Abstract
Terminal deoxynucleotidyl transferase (TdT) catalyzes the addition of nucleotides at the junctions of rearranging Ig and T cell receptor gene segments, thereby generating antigen receptor diversity. Ku is a heterodimeric protein composed of 70- and 86-kDa subunits that binds DNA ends and is required for V(D)J recombination and DNA double-strand break (DSB) repair. We provide evidence for a direct interaction between TdT and Ku proteins. Studies with a baculovirus expression system show that TdT can interact specifically with each of the Ku subunits and with the heterodimer. The interaction between Ku and TdT is also observed in pre-T cells with endogenously expressed proteins. The protein–protein interaction is DNA independent and occurs at physiological salt concentrations. Deletion mutagenesis experiments reveal that the N-terminal region of TdT (131 amino acids) is essential for interaction with the Ku heterodimer. This region, although not important for TdT polymerization activity, contains a BRCA1 C-terminal domain that has been shown to mediate interactions of proteins involved in DNA repair. The induction of DSBs in Cos-7 cells transfected with a human TdT expression construct resulted in the appearance of discrete nuclear foci in which TdT and Ku colocalize. The physical association of TdT with Ku suggests a possible mechanism by which TdT is recruited to the sites of DSBs such as V(D)J recombination intermediates.
Keywords: Double-strand break repair, VDJ recombination
V(D)J recombination is a site-specific event that generates the immune repertoire in mammalian lymphocytes (1). This event depends on the successful execution of a series of steps by protein factors, some of which function exclusively during V(D)J recombination, whereas others are also essential for double-strand break (DSB) repair (2). Rag1, Rag2, and terminal deoxynucleotidyl transferase (TdT) are lymphoid specific and function only during V(D)J recombination, whereas Ku70, Ku86, DNA-dependent protein kinase catalytic subunit (DNA-PKcs), XRCC4, and ligase IV are essential for both V(D)J recombination and DSB repair. Some of these factors interact with each other, suggesting a mechanism by which proteins are recruited to sites of recombination (3, 4).
TdT adds nucleotides in a template-independent manner at coding ends during recombination of Ig and T cell receptor gene segments, thereby expanding the diversity of antigen receptors. It exists as a 58-kDa protein, although smaller products of 55, 44, 32, and 12 kDa have been detected in cell extracts (5). The N terminus of TdT contains a conserved BRCT-like (BRCA1 C-terminal) sequence, a protein–protein interaction domain (6, 7) that was first identified in the breast cancer suppressor gene BRCA1 and subsequently found in 50 other proteins, some of which function in DNA repair and recombination (8, 9).
The Ku autoantigen is a heterodimer composed of 70- and 86-kDa subunits that form the DNA binding component of an associated 460-kDa DNA-PKcs (3). Ku86-deficient mice are able to initiate V(D)J recombination, but the intermediates of the cleavage reaction are not processed, resulting in accumulation of hairpin coding ends and blunt signal ends (10). The essential role of Ku in this process led us to ask whether it interacts directly with TdT, which modifies these intermediates by the insertion of nucleotides. Notably, the residual coding joints present in Ku86−/− mice were found to be devoid of N regions, suggesting that the Ku86 protein might regulate TdT activity in vivo (11). We have tested whether Ku and TdT interact directly in vitro and whether such interactions also occur in intact cells treated with a DNA-damaging agent.
Materials and Methods
Cell Culture.
The human Molt-4 (pre-T cell) lymphoblast cell line was maintained at 37°C in RPMI medium 1640 supplemented with 10% (vol/vol) FBS) and penicillin/streptomycin. Cos-7 cells were maintained at 37°C in DMEM supplemented with 10% (vol/vol) FBS. The Sf9 insect cell line was maintained at 27°C in Grace’s insect medium supplemented with 10% (vol/vol) FBS and penicillin/streptomycin.
Construction of Recombinant Baculovirus Constructs.
Full-length human TdT cDNA encoding the 58-kDa protein was cloned into the baculovirus transfer vector as described (12). The 44-kDa TdT lacking the N terminus was generated by PCR with the primers, 5′-CCTCTTCCCATGGACTATTCAGATAGCACCAACCCAGG-3′ and 5′-GACGAGCCTGATCAGGATCCTAGGCATTTCTTTCCCCACGG-3′. Recombinant baculoviruses expressing His-Ku70 and His-Ku86 have been described (13).
Antibodies.
For Western analysis, rabbit anti-human TdT polyclonal (5), mouse anti-polyhistidine (polyHis; Sigma), mouse anti-human Ku70, N3H10, and Ku86 (Sigma) mAbs were used. For coimmunoprecipitation, 5100/133D2 (anti-human TdT; ref. 12), N3H10 (anti-human Ku70; ref. 14), 111 (anti-human Ku86; ref. 15), 162 (anti-human Ku70/86; ref. 14), anti-polyHis, and polyclonal anti-human DNA-PKcs (PharMingen) antibodies were used. For immunofluorescence, rabbit anti-human TdT polyclonal and mouse anti-human Ku86 mAbs were used.
Western Blot Analyses.
Cell extracts were prepared in immunoprecipitation/lysis buffer (0.15 M NaCl/50 mM Tris, pH 7.5/0.3% NP-40/2 mM EDTA) with protease inhibitor mixture (Roche Molecular Biochemicals). Equal amounts were electrophoresed on SDS/PAGE gels, transferred to a poly(vinylidene difluoride) (PVDF) membrane, and probed with primary antibody followed by incubation in horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG secondary (1:4,000 dilution) antibodies. The blots were developed by using the enhanced chemiluminescence method (ECL kit, Amersham Pharmacia).
Immunoprecipitation of Lysates.
Lysates were prepared as described above. Equal amounts of the lysates were incubated overnight with antibody at 4°C. Protein A Sepharose beads were added, and the incubation was continued for 3 h. Bound proteins were released by boiling in 25 μl of 1.5× SDS/PAGE buffer and processed as described above.
Ethidium Bromide (EtBr) Treatment of Lysates.
The disruption of potential contaminating DNA was carried out by treatment with EtBr (16).
Double-Immunofluorescence and Microscopy.
Cos-7 cells were transfected with a human TdT expression construct (17) by using Lipofectamine (GIBCO/BRL). After etoposide treatment, cells were washed with PBS, treated with methanol for 30 min at −20°C, and rinsed briefly with ice-cold acetone as described by Haaf et al. (18). After three more washes with PBS, the coverslips were treated with PBS containing 0.2% Triton X-100 for 15 min at room temperature. The coverslips were washed three times with PBS, followed by incubation in blocking buffer [PBS/10% (vol/vol) FCS/3% (wt/vol) BSA] for 1 h at room temperature. The coverslips were then incubated with blocking solution containing mouse anti-Ku86 mAbs (1:400 dilution) and rabbit anti-TdT polyclonal antibodies (1:400 dilution) for 1 h. Cells were washed three times with PBS, followed by incubation in FITC-conjugated anti-mouse and rhodamine-conjugated anti-rabbit secondary antibodies for 1 h at room temperature. Cells were washed three times with PBS, followed by incubation in 4′,6-diamidino-2-phenylindole for 5 min. Coverslips were washed and mounted on glass slide in Vectashield mounting medium (Vector Laboratories). For detection of Ku-specific (FITC) fluorescence, cells were viewed with a filter set having an excitation filter of 480/20 nm, a dichroic beam splitter of 500 nm, and an emission filter of 515/30 nm. TdT specific fluorescence was visualized by using a filter set with an excitation filter of 560/20 nm, a dichroic beam splitter of 570 nm, and an emission filter of 590 nm (long pass). Fluorescence from 4′,6-diamidino-2-phenylindole in nuclei was viewed with the filter set having an excitation filter of 390/20 nm, a dichroic beam splitter of 420 nm, and an emission filter of 450/40 nm. The images were captured by using a slow-scan cooled charge-coupled device camera (exposure of 1.3–2 s).
Colocalization.
The images from individual nuclei were overlaid by using a computer algorithm (19). Briefly, a threshold was selected that obtained the data of interest and converted the image into a binary image. By using the filter “IP Boolean AND” operation, an image was generated that retained only the data that were common to both of the input images and thus provided the extent of colocalization. The filter “IP Math Subtract” function performed on each of the original input images gave the data that were unique for that particular image.
Results
Interaction of TdT and Ku70/86 Proteins.
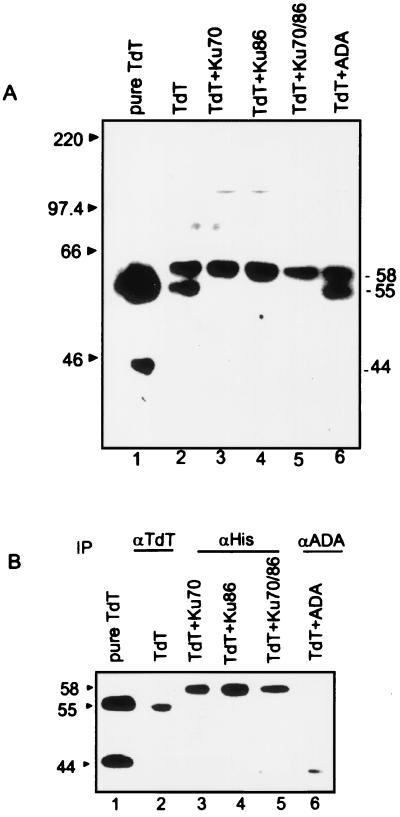
Genetic and biochemical data suggest that TdT and Ku may interact physically during V(D)J recombination in early lymphocyte development (11). To obtain direct evidence for complex formation, we chose to overexpress TdT and Ku70/86 and study their interaction (Fig. 1 A and B). Equivalent levels of expression of TdT were observed after infection with TdT alone or in combination with His-Ku70, His-Ku86, His-Ku70/86, or ADA (Fig. 1A, lanes 2–6). Purified TdT (Fig. 1A, lane 1) has undergone proteolysis from the 58-kDa form and migrates as two polypeptides with molecular masses of 55 kDa and 44 kDa, while retaining full catalytic activity. Infection of Sf9 cells with TdT baculovirus alone or in combination with ADA resulted in both the 58- and 55-kDa TdT proteins in cell extracts (Fig. 1A, lanes 2 and 6). However, coexpression of TdT either with Ku subunit alone or with both the subunits together seemed to stabilize TdT in the 58-kDa form completely (Fig. 1A, lanes 3–5).
Figure 1.
(A) Expression of TdT in Sf9 cells. Sf9 cells were infected with recombinant baculovirus expressing TdT (lane 2); TdT and His-Ku70 (lane 3); TdT and His-Ku86 (lane 4); TdT and His-Ku70/86 (lane 5); and TdT and adenosine deaminase (ADA; lane 6). The baculovirus constructs used for infections are indicated above the lanes. Lane 1 contains 30 ng of purified TdT. Extracts were made 72 h after infection, electrophoresed on SDS/10% PAGE, and transferred to a PVDF membrane. The blot was probed with rabbit anti-TdT polyclonal antibody at a 1:1,000 dilution and developed by using the enhanced chemiluminescence method. (B) Coimmunoprecipitation of Ku subunits with TdT. Approximately 50 μg of extract obtained from single, double, or triple coinfections was incubated with the anti-TdT 133D2 (lane 2), anti-polyHis (lanes 3–5), and anti-ADA N1D1 (lane 6) mouse mAbs. The immunoprecipitated complexes were resolved by SDS/10% PAGE, transferred to PVDF, and probed with rabbit anti-TdT polyclonal antibody (1:1,000 dilution). The blots were developed as described in A.
Equal amounts of cell extracts were analyzed for protein–protein interactions in coimmunoprecipitation experiments with anti-TdT (133D2; ref. 12) or anti-polyHis mAbs. Immunoprecipitation with the anti-polyHis mAb that recognizes the Ku-His-tagged protein coprecipitated the full-length 58-kDa TdT with each Ku subunit and with the heterodimer in the presence of 150 mM NaCl (Fig. 1B, lanes 3–5). TdT did not coimmunoprecipitate with ADA, indicating specificity of the TdT–Ku interaction (Fig. 1B, lane 6).
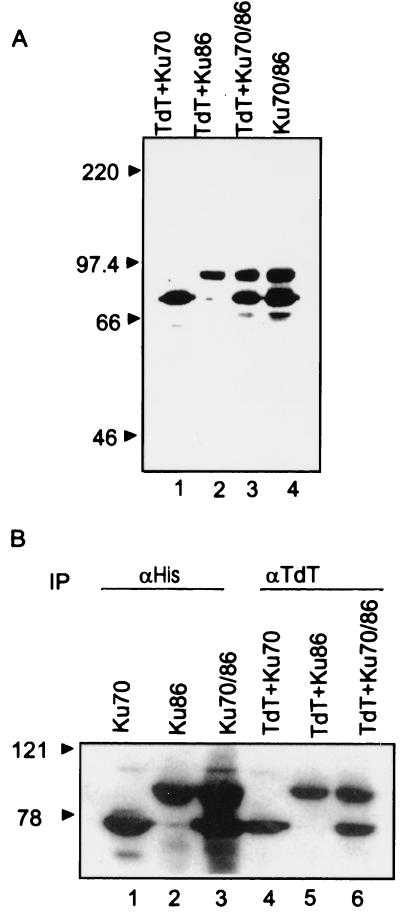
The interactions between TdT and Ku subunits were tested further by using TdT mAb for immunoprecipitation and anti-polyHis for Western analysis (Fig. 2). Anti-polyHis detected His-tagged Ku70 (Fig. 2A, lane 1) and His-tagged Ku86 (Fig. 2A, lane 2) but not TdT in cells coinfected with TdT and either of the Ku subunits alone or in combination (Fig. 2A, lane 3). Immunoprecipitation performed with the anti-TdT mAb (Fig. 2B, lanes 4–6) followed by Western analysis with the anti-polyHis antibody indicated that the Ku70 (Fig. 2B, lane 4), Ku86 (lane 5), or Ku70/86 (lane 6) proteins were present in the anti-TdT immunoprecipitates after coinfection with either or both of the Ku proteins, showing further that TdT complexes with the subunits independently as well as in combination.
Figure 2.
(A) Expression of Ku subunits in Sf9 cells. Sf9 cells were infected with recombinant baculovirus expressing TdT and His-Ku70 (lane 1); TdT and His-Ku86 (lane 2); TdT and His-Ku70/86 (lane 3); and Ku70/86 (lane 4). Extracts obtained 72 h after infection were electrophoresed on SDS/10% PAGE and transferred to a PVDF membrane. The blot was probed with anti-polyHis antibody (1:5,000 dilution) and developed by using the enhanced chemiluminescence method. (B) Coimmunoprecipitation of TdT with Ku subunits. Approximately 50 μg of extract obtained from single, double, or triple coinfection experiments was incubated with either the anti-polyHis (lanes 1–3) or anti-TdT 133D2 (lanes 4–6) mAbs. The immunoprecipitates were resolved by SDS/10% PAGE, transferred to PVDF membranes, and probed with anti-polyHis (1:5,000 dilution) antibody. The blots were developed as described in Fig. 1. IP, immunoprecipitation.
TdT–Ku Interaction in Molt-4 Lymphoblasts.
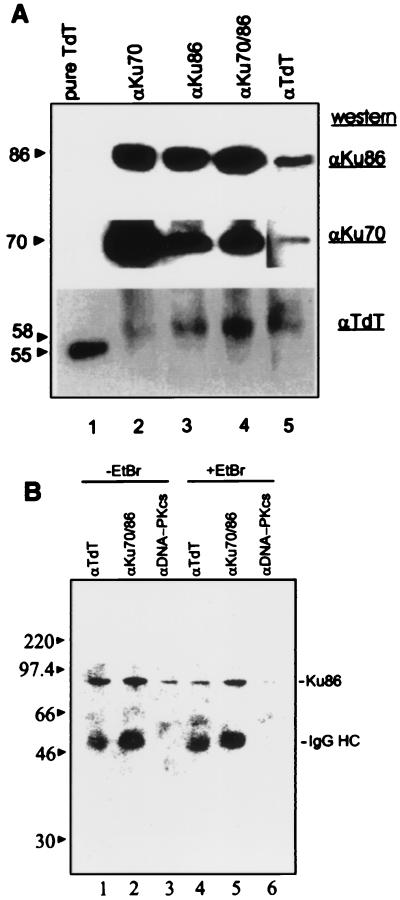
Based on the potential importance of the TdT–Ku interaction during V(D)J recombination and N region insertion in developing lymphocytes, we asked whether this interaction could also occur in human lymphoid cells. Immunoprecipitation of Molt-4 lymphoid cell extracts by using the anti-TdT mAb followed by Western analysis revealed a single band of 58 kDa (Fig. 3A Lower, lane 5). The 58-kDa protein was also coimmunoprecipitated with antibodies to Ku70 (Fig. 3A Lower, lane 2), Ku86 (Fig. 3A Lower, lane 3), and the Ku70/86 heterodimer (Fig. 3A Lower, lane 4). The blot was reprobed with mAbs specific for Ku70 or Ku86. Ku70 and Ku86 were immunoprecipitated with anti-Ku mAbs (Fig. 3A Middle and Top, lanes 2–4) as well as the anti-TdT antibody (lane 5). This result suggests that TdT and Ku associate in human lymphoid cell extracts and that the interaction is not an artifact of overexpression in Sf9 cells.
Figure 3.
(A) TdT–Ku interaction in Molt-4 lymphoblasts. Molt-4 lymphoid cell extracts (500 μg) were incubated with mAbs specific for Ku 70 (N3H10), Ku86 (111), Ku70/86 (162), or TdT (5100) as indicated above the lanes. Immunoprecipitated complexes were analyzed as described above. The blot was serially probed with rabbit anti-TdT (1:500 dilution; Bottom), mouse anti-Ku70 (1:1 dilution; Middle), or mouse anti-Ku86 antibodies (1:4,000 dilution; Top). The blot had to be exposed for a longer time to obtain a signal corresponding to Ku86. To prevent overexposure of other lanes, a separate exposure was obtained and included for lane 5. (B) Effect of EtBr on TdT–Ku interactions. Protein complexes were immunoprecipitated in the absence (lanes 1–3) or presence (lanes 4–6) of 100 μg/ml EtBr with anti-TdT, anti-Ku70/Ku86 heterodimer, and anti-DNA-PKcs mAbs (lanes 1 and 4, 2 and 5, 3 and 6, respectively) from Molt-4 cell extracts, resolved by SDS/PAGE, and analyzed by Western blotting by using anti-Ku86 as described in A.
TdT and Ku Interaction Is DNA Independent.
As TdT and Ku are both capable of binding DNA, it is important to determine whether the observed interaction of TdT and Ku could be due to the presence of contaminating DNA in the cell lysates. Previous work showed that the Ku and DNA-PKcs interaction is DNA dependent, whereas heterodimerization between Ku70 and Ku86 occurs in the absence of DNA (3, 13). To test for DNA-dependent interactions, immunoprecipitations were performed by using Molt-4 cell extracts treated with 100 μg/ml EtBr (16). TdT, Ku70/Ku86, and DNA-PKcs immunoprecipitates were tested for the presence of Ku86 by Western analysis (Fig. 3B). In the absence of EtBr, Ku86 was found to be coimmunoprecipitated with TdT, Ku70, and DNA-PKcs (Fig. 3B, lanes 1–3). However, in the presence of EtBr, the Ku and DNA-PKcs interaction was specifically disrupted, whereas the TdT–Ku86 and Ku86–Ku70 interactions remained intact (Fig. 3B, lanes 4–6).
Additional evidence for DNA-independent interactions between Ku and TdT is the association between TdT and each Ku subunit. The Ku86 subunit alone does not bind DNA, and the Ku70–DNA interaction is weak in the absence of Ku86 (20). Further, it has been shown that at 500 mM NaCl, DNA dissociates from Ku (21), whereas the TdT–Ku86 complex is stable at this salt concentration.
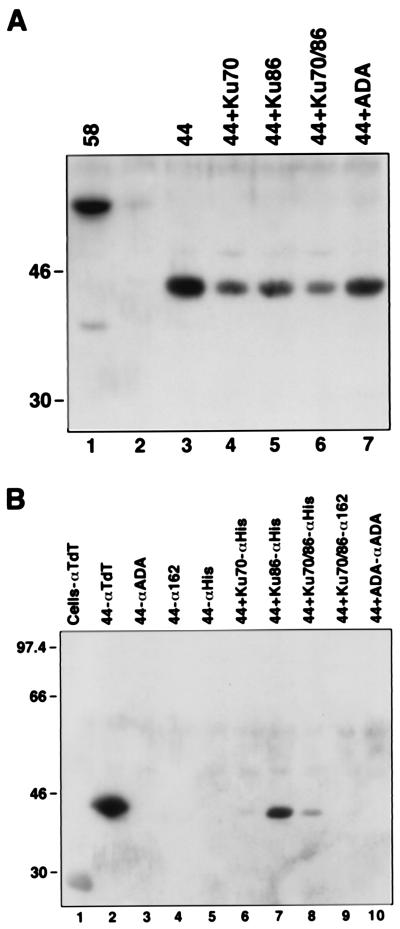
The N-Terminal 131 Amino Acids of TdT Are Essential for the TdT–Ku Interaction.
To identify the domains in TdT that interact with Ku, we constructed a 44-kDa truncated form of TdT in which 131 amino acids at the N terminus containing the BRCT domain were deleted. This form of TdT, which is also generated by proteolysis of full-length TdT in cell extracts, retains the DNA and nucleotide binding domains and is fully active. Sf9 insect cells were infected with 44-kDa TdT, in conjunction with His-tagged Ku70, Ku86, or both Ku70 and Ku86. The expression of each protein was monitored by Western analysis (Fig. 4A). Coimmunoprecipitation with the anti-polyHis mAb followed by Western blotting for TdT revealed that 44-kDa TdT strongly and specifically interacted with the Ku86 subunit (Fig. 4B, lane 7), whereas a weaker or no interaction was observed in cells expressing the Ku heterodimer (Fig. 4B, lanes 8 and 9). The interaction with the 70-kDa subunit was lost (Fig. 4B, lane 6), suggesting that the BRCT domain is necessary for TdT–Ku70 and TdT–Ku heterodimer interaction.
Figure 4.
(A) Expression of 44-kDa N-terminal truncation of TdT. Extracts from uninfected (lane 2) or baculovirus-infected Sf9 cells expressing 44-kDa TdT (lane 3), 44-kDa TdT with Ku70 (lane 4), 44-kDa TdT with Ku86 (lane 5), 44-kDa TdT with Ku70/86 heterodimer (lane 6), or 44-kDa TdT with ADA (lane 7) were analyzed by Western blotting by using rabbit polyclonal anti-TdT. (B) Coimmunoprecipitation of 44-kDa TdT with Ku86. Extracts from uninfected Sf9 cells (lane 1) or cells infected with baculovirus expressing 44-kDa TdT (lanes 2–5), 44-kDa TdT with Ku70 (lane 6), 44-kDa TdT with Ku86 (lane 7), 44-kDa TdT with Ku70/86 (lanes 8 and 9), or 44-kDa TdT with ADA (lane 10) were immunoprecipitated with anti-TdT (lane 2), anti-ADA (lanes 3 and 10), anti-Ku heterodimer (anti-162; lanes 4 and 9), or anti-His (anti-Ku; lanes 5–8) and analyzed as described for A.
Colocalization of TdT and Ku After DNA Damage.
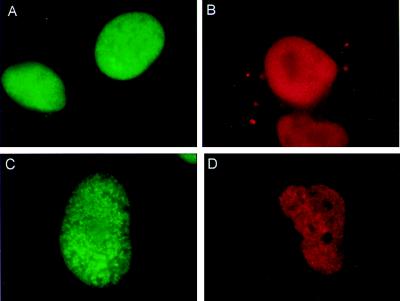
The interaction of TdT with Ku was also examined in intact cells by using a double-immunofluorescence technique (22). To overcome the limitation of the small size of Molt-4 nuclei and low level of TdT expression, we performed the experiments in Cos-7 cells containing endogenous Ku that were transiently transfected with a human TdT expression construct (17). Expression of both Ku and TdT in vivo was confirmed by staining cells with anti-Ku and anti-TdT primary antibodies, followed by incubation with sheep FITC-conjugated anti-mouse (anti-Ku, green fluorescence) and goat-rhodamine conjugated anti-rabbit (anti-TdT, red fluorescence) IgG secondary antibodies. Under normal growth conditions, both proteins were uniformly distributed in the nucleus (Fig. 5 A and B). To test whether the induction of DSBs, similar to those that occur during V(D)J recombination (1), would alter the distribution of the TdT and Ku proteins, Cos-7 cells transfected with TdT were treated with etoposide, a topoisomerase II inhibitor. Untreated cells did not have clear foci or colocalization of Ku and TdT (Table 1). After 3 h of treatment with 2 μM etoposide, numerous distinct bright spots or foci of TdT and Ku immunofluorescence were observed; they were consistently visible in three independent experiments (Fig. 5 C and D). Quantitative analysis revealed that, after 3 h, ≈10% of the cells were positive for Ku foci and ≈14% were positive for TdT foci when examined individually for each of the proteins. At 5 h, there was an increase in the number of nuclei showing foci (Table 1). Greater than 80% of TdT foci-positive nuclei were also positive for Ku foci at both 3 and 5 h after etoposide treatment. TdT and Ku foci were not detectable in the nucleolus, where TdT staining has also been found to be weak (23).
Figure 5.
Double-immunoflourescence analysis of Cos-7 cells transfected with human TdT: effect of etoposide treatment. Cos-7 cells transfected with the human TdT expression construct were treated with 2 μM of etoposide in DMSO (C and D) or DMSO alone (A and B) for 3–5 h and fixed. Cells were stained with FITC-αKu (green) or rhodamine-αTdT (red). Both proteins are present only in the nucleus.
Table 1.
Induction of Ku and TdT foci by etoposide treatment in Cos-7 cells
| Etoposide treatment, h | No. of nuclei examined for Ku | Percentage of nuclei with Ku foci | No. of nuclei examined for TdT | Percentage of nuclei with TdT foci |
|---|---|---|---|---|
| No treatment | 216 | 0 | 107 | 0 |
| 3 | 258 | 10 | 209 | 14 |
| 5 | 383 | 14 | 229 | 21 |
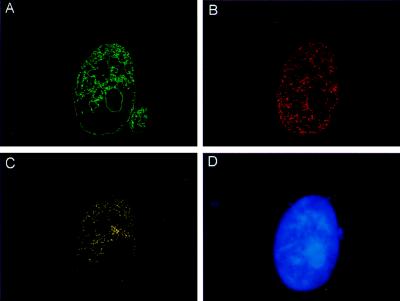
The possibility that TdT and Ku were interacting after the induction of DSBs was tested by measurement of colocalization of foci. Images of the nuclei were acquired first with FITC (green) filters (Ku localization) and subsequently with rhodamine (red) filters (TdT localization). Images obtained from the same nucleus that had been stained with both antibodies were analyzed by using a computer program designed to measure the extent of colocalization (19). Results of analyses from five nuclei showed that colocalized TdT and Ku foci were scattered at many locations in the nucleus. A representative nucleus is shown in Fig. 6. The Ku and TdT foci that are not colocalized (Fig. 6 A and B) in comparison with clearly colocalized regions (Fig. 6C) are shown. Quantitative analysis of individual nuclei based on the number of overlapping pixels revealed that 10–25% of TdT foci were colocalized with Ku in response to etoposide treatment. Importantly, as the program performs pixel by pixel analysis, only those pixels that are coincident in both images are used to obtain the colocalization image, avoiding any possibility of fortuitous colocalization. A similar extent of TdT–Ku colocalization was observed in all nuclei that contained TdT or Ku DSB-induced foci.
Figure 6.
Colocalization of TdT and Ku after etoposide treatment. Images obtained from the same treated nucleus stained for FITC-αKu (green; A) and rhodamine-αTdT (red; B) were digitized. By using the image processing tool kit (20) in adobe photoshop 4.01, the foci that were not colocalized for both Ku or TdT were subtracted (A and B), and only Ku and TdT foci that were truly colocalized remained (yellow; C). (D) DNA counterstained with 4′,6-diamidino-2-phenylindole (blue).
Discussion
We have shown that full-length TdT interacts directly with the Ku70/86 heterodimeric protein, as well as with each subunit independently, and that this interaction stabilizes TdT against proteolysis. In contrast to the Ku–DNA-PKcs interaction, the interaction of TdT with the Ku subunits and with the heterodimeric protein is DNA independent. Of particular interest is the observation that the N-terminal 131 amino acids of TdT that encompass a BRCT domain were required for the interaction of TdT with Ku70 but not with the Ku86 subunit or with the heterodimeric protein. The BRCT motif is found in a number of DNA repair proteins and seems to mediate protein–protein interactions, such as those found in XRCC1 and DNA ligase III, where it exists as an independent folding region (8, 9). It seems likely that this evolutionarily conserved motif may play an important role in recruiting TdT to the ends of DNA DSBs.
Our data on TdT–Ku interactions have significant implications for V(D)J recombination. Ku protein binds at the sites of DNA DSBs and is required for the resolution of signal and coding junction intermediates generated during the recombination process (10). We and others have shown that TdT mediates N region insertions at both signal and coding junctions (17, 24). Our results indicate that TdT may be recruited to the recombination sites by direct contacts with Ku. This interaction may modulate TdT activity at the recombination intermediates. Support for this hypothesis comes from the recent direct demonstration that Ku86−/− mice lack N region insertions at coding joints (11).
Recent experiments have identified regions of the Ku70 and 86 subunits that are required for heterodimerization. The C-terminal half of Ku86 (amino acids 334–732) containing a proline-rich domain is essential for its dimerization to Ku70 and subsequent DNA binding activity (25). Truncation of the C-terminal portion of Ku86 in an HL-60 cell line resulted in diminished DNA binding and heterodimerization abilities as well as in defective interactions with DNA-PKcs (26). The C-terminal portion of the p70 subunit directly binds double-stranded DNA in the absence of heterodimerization with p86, and two distinct dimerization domains at the N terminus and at amino acids 430–482 have been identified (20). Functional roles for leucine zipper motifs found in both p70 and p86 have not been identified (15, 25). Although direct experimental data are lacking and TdT does not have a canonical leucine zipper region, it is conceivable that the Ku leucine zippers could play a role in DNA-PKcs interactions or in TdT–Ku interactions.
Further evidence for a model in which TdT and Ku interact at sites of DSBs is the formation of colocalized nuclear foci of TdT and Ku proteins in cells induced to undergo DSBs. These results provide in vivo evidence for the formation of TdT and Ku complexes in cells treated with DSB-inducing agents. A study by Maser et al. (22) shows that two of the DSB repair proteins, human Mre11 and human Rad50, colocalize in discrete nuclear foci after treatment with agents that induce DSBs, whereas Haaf and coworkers (18, 27) show that another DNA repair and recombination protein, human Rad51, localizes in nuclear foci after exposure to γ-irradiation or etoposide treatment. Foci of Ku and TdT colocalized in greater than 80% of the cells, and the extent of colocalization was 10–25%. Recent data show that human Rad51 and RPA (Replication Protein A) foci were colocalized and that >10% of human Rad51 foci were localized at sites of DNA damage (28). We postulate that the TdT–Ku interaction may exist in a dynamic equilibrium, possibly relating to the extent of TdT proteolysis. The in vivo interaction of TdT with Ku suggests that polymerization by TdT occurs at exogenously induced DSBs. Whether this interaction occurs before the recruitment of a preformed complex of TdT–Ku to DNA break sites or by interaction after independent protein localization at DNA ends is currently unclear. It is also unknown whether the localization of TdT at DSB sites could further sensitize TdT-expressing pre-B or pre-T cells to DSB-inducing agents, such as ionizing radiation by catalyzing nucleotide additions at these sites (29).
Recent reports suggest that the Saccharomyces cerevisiae complex of ScRAD50, ScMre11, and ScXRS2 participate in Ku-dependent DNA DSB repair. One function of this trimeric complex could be to serve as a bridge between Ku and other members of the nonhomologous end-joining pathway (30). It will be important to determine whether human TdT and Ku are shared components in a DSB repair pathway of developing lymphocytes that may also include the human homologs of Mre11, p95, and Rad50. Furthermore, it will be of interest to pursue the potential for detrimental effects of TdT activity at sites of DSBs not involved in V(D)J recombination in TdT-positive cell lines.
Acknowledgments
We thank Dr. Mark Knuth (Promega) for supplying mAb N3H10. This work was supported by National Institutes of Health Grants CA34085 (to B.S.M), CA67156 (to D.H.S), and R01AR40391 (to W.H.R). J. Wang was supported by a postdoctoral fellowship from the Arthritis Foundation.
Abbreviations
- TdT
terminal deoxynucleotidyl transferase
- DSB
double-strand break
- DNA-PKcs
DNA-dependent protein kinase catalytic subunit
- BRCT
BRCA1 C terminal
- PVDF
poly(vinylidene difluoride)
- EtBr
ethidium bromide
- ADA
adenosine deaminase
- polyHis
polyhistidine
References
- 1.Tonegawa S. Nature (London) 1983;302:575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- 2.Chu G. J Biol Chem. 1997;272:24097–24100. doi: 10.1074/jbc.272.39.24097. [DOI] [PubMed] [Google Scholar]
- 3.Gottlieb T M, Jackson S P. Cell. 1993;72:131–142. doi: 10.1016/0092-8674(93)90057-w. [DOI] [PubMed] [Google Scholar]
- 4.Leber R, Wise T W, Mizuta R, Meek K. J Biol Chem. 1998;273:1794–1801. doi: 10.1074/jbc.273.3.1794. [DOI] [PubMed] [Google Scholar]
- 5.Fuller S A, Philips A, Coleman M S. Biochem J. 1985;231:105–113. doi: 10.1042/bj2310105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koonin E V, Altschul S F, Bork P. Nat Genet. 1996;13:266–268. doi: 10.1038/ng0796-266. [DOI] [PubMed] [Google Scholar]
- 7.Callebaut I, Mornon J P. FEBS Lett. 1997;400:25–30. doi: 10.1016/s0014-5793(96)01312-9. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Morera S, Bates P A, Whitehead P C, Coffer A I, Hainbucher K, Nash R A, Sternberg M J E, Lindahl T, Freemont P S. EMBO J. 1998;17:6404–6411. doi: 10.1093/emboj/17.21.6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nash R A, Caldecott K W, Barnes D E, Lindahl T. Biochemistry. 1997;36:5207–5211. doi: 10.1021/bi962281m. [DOI] [PubMed] [Google Scholar]
- 10.Zhu C, Bogue M A, Lim D S, Hasty P, Roth D B. Cell. 1996;86:379–389. doi: 10.1016/s0092-8674(00)80111-7. [DOI] [PubMed] [Google Scholar]
- 11.Bogue M A, Wang C, Zhu C, Roth D B. Immunity. 1997;7:37–47. doi: 10.1016/s1074-7613(00)80508-7. [DOI] [PubMed] [Google Scholar]
- 12.Yang B, Gathy K N, Coleman M S. J Biol Chem. 1994;269:11859–11868. [PubMed] [Google Scholar]
- 13.Wang J, Dong X, Myung K, Hendrickson E A, Reeves W H. J Biol Chem. 1998;273:842–848. doi: 10.1074/jbc.273.2.842. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Satoh M, Pierani A, Schmitt J, Chou C H, Stunnenberg H G, Roeder R G, Reeves W H. J Cell Sci. 1994;107:3223–3233. doi: 10.1242/jcs.107.11.3223. [DOI] [PubMed] [Google Scholar]
- 15.Reeves W H. J Exp Med. 1985;161:18–39. doi: 10.1084/jem.161.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai J S, Herr W. Proc Natl Acad Sci USA. 1992;89:6958–6962. doi: 10.1073/pnas.89.15.6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gangi-Peterson L, Sorscher D H, Reynolds J W, Mitchell B S. J Clin Invest. 1999;103:833–841. doi: 10.1172/JCI4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haaf T, Raderschall E, Reddy G, Ward D C, Radding C M, Golub E I. J Cell Biol. 1999;144:11–20. doi: 10.1083/jcb.144.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russ J. In: Image Processing Handbook. Russ J, editor. Boca Raton, FL: CRC; 1995. pp. 407–414. [Google Scholar]
- 20.Wang J, Dong X, Myung K, Hendrickson E A, Reeves W H. J Biol Chem. 1998;273:31068–31074. doi: 10.1074/jbc.273.47.31068. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Satoh M, Chou C H, Reeves W H. FEBS Lett. 1994;351:219–224. doi: 10.1016/0014-5793(94)00863-9. [DOI] [PubMed] [Google Scholar]
- 22.Maser R S, Monsen K J, Nelms B E, Petrini J H. Mol Cell Biol. 1997;17:6087–6096. doi: 10.1128/mcb.17.10.6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jani P, Verbi W, Greaves M F, Bevan D, Bollum F. Leukemia Res. 1983;17:17–29. doi: 10.1016/0145-2126(83)90054-1. [DOI] [PubMed] [Google Scholar]
- 24.Gauss G H, Lieber M R. Mol Cell Biol. 1995;16:258–269. doi: 10.1128/mcb.16.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu X, Lieber M R. Mol Cell Biol. 1996;16:5186–5193. doi: 10.1128/mcb.16.9.5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharkey M, Graba Y, Scott M P. Trends Genet. 1997;13:145–151. doi: 10.1016/s0168-9525(97)01096-2. [DOI] [PubMed] [Google Scholar]
- 27.Haaf T, Golub E I, Reddy G, Radding C M, Ward D C. Proc Natl Acad Sci USA. 1995;92:2298–2302. doi: 10.1073/pnas.92.6.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raderschall E, Golub E I, Haaf T. Proc Natl Acad Sci USA. 1999;96:1921–1926. doi: 10.1073/pnas.96.5.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sale E J, Neuberger M S. Immunity. 1998;9:859–869. doi: 10.1016/s1074-7613(00)80651-2. [DOI] [PubMed] [Google Scholar]
- 30.Boulton S J, Jackson S P. EMBO J. 1998;17:1819–1828. doi: 10.1093/emboj/17.6.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]