Abstract
Alcoholism is a complex, multifactorial disorder involving problematic ethanol ingestion; it results from the interplay between genetic and environmental factors. Personality, likewise, is formed from a combination of inherited and acquired influences. Because selected dimensions of emotional temperament are associated with distinct neurochemical substrates contributing to specific personality phenotypes, certain aspects of abnormal emotional traits in alcoholics may be inherited. Emotions involve complex subjective experiences engaging multiple brain regions, most notably the cortex, limbic system, and cerebellum. Results of in vivo magnetic resonance imaging and post-mortem neuropathological studies of alcoholics indicate that the greatest cortical loss occurs in the frontal lobes, with concurrent thinning of the corpus callosum. Additional damage has been documented for the amygdala and hippocampus, as well as in the white matter of the cerebellum. All of the critical areas of alcoholism-related brain damage are important for normal emotional functioning. When changes occur in these brain regions, either as a consequence of chronic ethanol abuse or from a genetic anomaly affecting temperament and/or a vulnerability to alcoholism, corresponding changes in emotional functions are to be expected. In alcoholics, such changes have been observed in their perception and evaluation of emotional facial expressions, interpretation of emotional intonations in vocal utterances, and appreciation of the meaning of emotional materials.
Keywords: alcoholism, personality, emotional dysfunction, genetic influences
Introduction
Emotions engage strong mental and affective states giving rise to intense feelings, both positive and negative. Emotions generally are considered to be separate from cognition, although it is recognized that emotions can directly influence various aspects of mental function, and vice versa (Cahill 2003). The brain contributes to emotional functioning in a coordinated fashion that involves multiple systems of the body (Keltner and Shiota 2003). The emotional changes accompanying long-term chronic alcoholism cover a broad spectrum. Some of these changes, eg, apathy and emotional flatness, are reminiscent of those seen in patients with bilateral frontal lobe damage (Lezak 1995; Moselhy et al 2001; Di Lazzaro et al 2004) or in patients with right-hemisphere damage (Kaplan 1988). Other abnormalities are subtle. For example, alcoholics may make atypical judgments regarding the nature of facial emotional expressions (Oscar-Berman et al 1990; Townshend and Duka 2003) or intonations of emotional utterances (Monot et al 1994; Wildgruber et al 2002; Gandour et al 2003). It also has been suggested that alcoholism may involve an underlying neurocognitive deficit in the capacity to comprehend emotional information (Loas et al 2000; George et al 2001; Ravaglia et al 2002; Townshend and Duka 2003). Furthermore, an individual’s genetic history can impact both a tendency toward alcoholism and the development of anomalies in areas of the brain involved in emotional processing (Dick and Foroud 2003; Yamasaki et al 2003; Bowirrat and Oscar-Berman 2005). Taken together, these various views reflect considerable uncertainty about the nature of emotional changes in alcoholism. Whatever the mechanism(s), however, it is clear that alcoholism can damage the brain, and emotional abnormalities in alcoholics can interfere with healthy interpersonal and work-related relationships. In the present paper, we review research that has examined the effects of long-term alcoholism on brain systems involved in emotional functions. We conclude that abnormal emotional traits in alcoholics, some of which may be inherited, are associated with corresponding abnormalities in frontal, limbic, and cerebellar brain systems.
Genetics of temperament in relation to alcoholism
Genetic knowledge provides us not only with a molecular explanation for various behaviors, but also with the realization that we all carry vulnerabilities to medical and psychiatric disorders. Genes are segments of DNA that consist of codes for proteins the body makes and uses to run itself on a daily basis. Many of those proteins create physical characteristics (eg, eye color). Other genes contain the instructions for proteins that function as chemical messengers in the body. Some hormones are chemical messengers that can have powerful effects on emotions. Hormones are molecules that fit into specific receptor sites on living tissues, and that cause those tissues to respond in a certain way. Many hormones travel by way of the blood stream a great distance from where they are manufactured to the target organ they affect. However, other hormones often are manufactured and stored in the tissue where they are later used. A common hormone with which many people are familiar is epinephrine (adrenaline) – the “fight-or-flight” hormone. When a person is frightened, scared, or severely startled, this hormone is released by the adrenal glands into the blood stream. Epinephrine fits into target sites on the heart, brain, iris, smooth muscle tissue of the intestine, and other organs. The action of this hormone is to increase heart rate, which prepares a person for a proper response, eg, providing additional oxygen to leg muscles so the person can run fast if necessary. At the same time, epinephrine dilates the eyes, reduces circulation to the stomach and intestine, and increases circulation to the limbs (which can make a person feel physically agitated or “jumpy”).
Other hormones, having a target area primarily in the brain, may affect emotion more directly. These include dopamine, serotonin, and endorphins, and they have powerful effects on emotions ranging from chronic depression to euphoria. Because these hormones can be used up faster than they are produced, sometimes people self medicate in order to feel particular emotions. For example, prolonged physical or emotional stress can lead to the use of substitute substances such as alcohol, caffeine, or drugs of abuse. Whether an individual turns to drugs for emotional stimulation depends upon numerous factors, including genetic background and environmental circumstances.
Although it is well established that personality traits are heritable (Loehlin 1992; Bouchard 1994; Jang et al 1996), most theories of personality do not attempt to identify the specific genes involved. Cloninger and colleagues (1993), however, proposed a psychobiological model of personality that purports to map personality at the genetic level. They proposed that temperament and character traits can be described in terms of specific psychological factors, and that these traits are associated with genetically determined neurochemical substrates. An obvious implication of this model is that genes associated with neurotransmitters are related to the hypothesized temperament traits. Another implication is that traits hypothesized to have a shared genetic basis should covary at the phenotypic level.
In line with these ideas, Cloninger and his colleagues developed the Temperament and Character Inventory (Cloninger et al 1994), a seven-factor scale measuring four temperament and three character dimensions. Cloninger and his colleagues claimed that the temperament dimensions of novelty seeking, harm avoidance, reward dependence, and persistence are genetically homogeneous and that two of them are associated with distinct neurochemical substrates: novelty seeking with dopamine, and harm avoidance with serotonin. The model also identified three character dimensions called self-directedness, cooperativeness, and self-transcendence, which are based on social goals and values. According to Cloninger and colleagues (1996), the psychobiological model accounts for the genetic basis of the personality phenotype, whereas alternative models of personality comprise genetically and environmentally heterogeneous factors.
There is some support for Cloninger’s ideas from the field of molecular psychiatry and the mapping of candidate genes for dimensions of human personality traits. In three studies (Benjamin et al 1996; Ebstein et al 1996; Lesch et al 1996), the dimension of novelty seeking (which includes exploratory excitability, impulsiveness, extravagance, and disorderliness) was linked to the seven-repeat allele (or long form) of the 16-amino-acid polymorphism of the D4 dopamine receptor gene (DRD4), and in another study harm avoidance was associated with the short form of a functional polymorphism (44-base-pair insertion or deletion) in the serotonin-transporter-linked promoter region (5-HTTLPR). These results provided empirical support for Cloninger’s claim that the temperament dimensions of novelty seeking and harm avoidance have an identifiable genetic basis.
Although replications have been reported (Ebstein et al 1997; Ono et al 1997; Noble et al 1998; Ricketts et al 1998; Katsugari et al 1999; Strobel et al 1999; Keltikangas-Jarvinen et al 2003), there also have been numerous failures to replicate the associations with DRD4 (Malhotra et al 1996; Gelernter et al 1997; Sander et al 1997; Pogue-Geile et al 1998; Sullivan et al 1998; Ekelund et al 1999; Kühn et al 1999) and 5-HTTLPR (Ebstein et al 1997; Gelernter et al 1998; Mazzanti et al 1998; Kumakiri et al 1999). Other studies also failed to replicate any positive involvement in both neurotransmitters, ie, between the DRD4 polymorphism and novelty seeking, and between the 5-HTTLPR polymorphism and harm avoidance (Ball et al 1999; Herbst et al 2000). Such failures to replicate raise the question of whether the temperament-character model adequately taps the genetic architecture of personality.
Evidence from twin studies supports the idea that at least 40% of the addictions to alcohol, tobacco, and other drugs have genetic influences (Merikangas 1998; Tsuang et al 1998; McGue 1999; Karkowski et al 2000; Theodore et al 2003; Uhl and Grow 2004). For example, Jacob et al (2003) reported that offspring of monozygotic and dizygotic twins with a history of alcohol dependence were found to exhibit alcohol abuse or addiction more frequently than offspring of nonalcoholic fathers, and offspring of an alcohol-abusing monozygotic twin whose co-twin was alcohol dependent were more likely to be alcohol-dependent than offspring of nonalcoholic twins. However, in the absence of paternal alcoholism, offspring with high genetic risk (the unaffected father’s co-twin is alcoholic) showed lower rates of alcoholism than children of alcoholics (Jacob et al 2003). Genome scans have identified multiple addiction vulnerability loci, but no regions that seem to contain genes of major effect in alcoholics or poly-substance abusers (Long et al 1998; Foroud and Li 1999; Uhl et al 2001).
A large collaborative research program on the genetics of alcoholism (COGA), involving several institutions in the USA, seeks to identify genes contributing to alcoholism and related traits (ie, phenotypes), including comorbid psychiatric conditions. COGA investigators have found an increased prevalence of depressive syndromes in alcoholics. In particular, the combination of alcoholism and depression tends to cluster in families (Bierut et al 2002; Nurnberger et al 2002). Comorbid alcoholism and depression occurred substantially more often in first-degree relatives of COGA participants with alcoholism than in relatives of nonalcoholic control participants. Based on these data, COGA investigators defined three phenotypes: alcoholism (ALC); alcoholism and depression (AAD); and alcoholism or depression (AorD). The data were analyzed to determine whether the phenotypes were linked to specific chromosomal regions. These analyses have identified several chromosomal regions, particularly on chromosomes 1 and 4 that appear to be linked to alcohol-related phenotypes (Bierut et al 2002; Nurnberger et al 2002). In addition, increased allele sharing was seen near two markers called D1S1648 and D1S1588 between 100 and 110 centi-Morgan (Nurnberger et al 2002). The same portion of chromosome 1 that exhibited linkage with the AorD phenotype also showed suggestive linkage with the ALC phenotype alone (Reich et al 1998). This suggests that a gene or genes on chromosome 1 may predispose some people to depression and others to alcoholism.
Candidate gene and whole-genome linkage analyses on alcoholism and antisocial alcoholism (alcoholism plus antisocial personality disorder or intermittent explosive disorder) were performed in population isolates consisting of Finnish families, among whom probands were alcoholic offenders, and also in a large Southwestern American Indian family (Reich et al 1998; Nurnberger et al 2002). Results included strong evidence for genetic influences in antisocial alcoholism. Linkage was found at chromosomes 11p (location of DRD2 dopamine receptor), 4p (GABAA cluster), and 4q (ADH cluster), as well as sibpair linkage to the 5HT1B receptor gene previously implicated by mouse 5HT1B knockout studies (Adamson et al 1995). In recent years, there also have been indications that two serotonin genes, 5-HT1B and tryptophan hydroxylase (TPH), may be linked to the impulsive or antisocial dimensions of behavior in alcoholics (Lappalainen et al 1998; Nielsen et al 1998; Kühn et al 1999).
Goldman and colleagues (Goldman 1996; Enoch and Goldman 2001; Heinz et al 2001; Gray and McNaughton 2002) reported the results of a meta-analysis study in which they examined the correlation between central serotonergic neurotransmission and three behavior patterns that are relevant for alcoholism: disinhibition (impulsive aggression), negative mood states (such as anxiety and depression), and a low response to alcohol. The authors noted that the neurotransmitter serotonin (5-HT3) is very likely influenced by genetics and early stress experiences, as well as alcohol itself. Serotonergic dysfunction has been linked to a number of psychiatric disorders, as well as the development and maintenance of excessive alcohol consumption and alcoholism. Serotonin plays a role in temperature control and sleep and also affects impulse control and behavior inhibition. Circuits that depend on serotonin are also vital to a person’s sense of wellbeing and are involved in reward, allowing the use of selective serotonin reuptake inhibitors to alleviate feelings of anxiety and dysphoria.
Serotonergic dysfunction also seems to be a cause of negative mood states, namely anxiety and depression (Barr et al 1994; Artigas 1995; Mann et al 1996). Among alcoholics, the association between serotonergic dysfunction and depression is less well established. Some genetic linkage and brain imaging studies, however, have suggested that a reduced availability of serotonin transporters is associated with anxiety and depressed mood states among alcoholics, patients with major depression, and control subjects (Malison et al 1998; Mazzanti et al 1998; Rosenthal et al 1998; Heinz et al 2001). Some studies indicate that the association between a low serotonin turnover rate and aggressive behavior may be mediated by negative emotions, such as feeling insecure and threatened. Virkunnen and colleagues (1994) observed that alcoholics with low serotonin turnover and high aggressiveness suffer from increased anxiety. A reduction in central serotonin turnover has been observed in heterogeneous groups of individuals, eg, alcoholics, violent criminals, and fire-setters. It has been suggested that “impulsive aggression” is the behavioral characteristic common to all of these individuals (Kruesi et al 1990; Virkunnen et al 1994).
Alcohol stands out as the one agent that is consistently associated with increased risk-taking, criminal activity, and aggressive or violent behavior more than many other drugs (Ensor and Godfrey 1993; Taylor and Chermack 1993; Freemantle 1993; Knight and Godfrey 1993; Seto and Barbaree 1995; Galanter 1997; Lanza-Kaduce et al 1997; Giancola et al 2003; Miczek et al 2003; Lane et al 2004). Research overwhelmingly indicates that children of alcoholics (who are more likely to develop significant alcohol problems) also develop behavioral problems more often than children of nonalcoholics. Miranda et al (2002) used an emotion-modulated startle paradigm to test the hypothesis that young adults with positive paternal history of alcoholism may have altered emotional reactivity to environmental cues. Alcohol or other substance abusers tended to have specific kinds of conduct disorder traits when they were children (eg, attention deficit disorder, hyperactivity, rule breaking, and poor response to discipline), and later they developed antisocial personality disorder. A lack of response to social censure and physical punishment is atypical, and there is emerging evidence that the systems embedded in the brain that respond to emotionally significant events may be lacking in some individuals who are unable to respond normally to external events (Monot et al 2001; Miranda et al 2002).
Among the actions of alcohol on several ligand-gated ionophores such as NMDA, 5-HT3, or cholinergic receptors, the positive modulation of the GABAA receptor appears to be of particular significance with regard to aggressive behavior (Miczek et al 2002, 2003). Moreover, the interactive effects of alcohol with neurosteroids and benzodiazepines appear to be mediated by action at modulatory sites on the GABAA receptor (Grobin et al 1998; Kumar et al 2004).
The first indication for a significant role of GABAA receptors in alcohol-heightened aggression was the effective blockade by benzodiazepine receptor antagonists. Pretreatment with the broad-spectrum antagonists flumazenil and ZK93426 prevented alcohol-heightened aggressive behavior in nonhuman animal models (Miczek, Barros, et al 1998; Miczek, de Almeida, et al 1998; Miczek et al 2001; de Almeida et al 2004). Furthermore, benzodiazepine agonists such as chlordiazepoxide can enhance the aggression-heightening effects of alcohol (Miczek and O’Donnell 1980). Alcohol effects on aggressive behavior depend not only on the molecular characteristics of the GABAA receptor, but also on its interactions with other positive modulators. In addition to benzodiazepines, alcohol interacts with neurosteroids, possibly at similar subunits of the GABAA receptor.
Emotional dysfunction and brain damage in alcoholism
Alcoholics have impairments in cognitive processing of emotional signals. They seem to know what to do in interpersonal situations, but their social skills are impaired, and many are unable to implement the strategies they recommend for themselves (Gaffney et al 1998). More specifically, they exhibit deficits in decoding affective prosody, a non-linguistic aspect of language that conveys emotion and attitude during discourse (Monot et al 2001). Monot et al (2001) reported that alcoholics were deficient in the ability to detect emotion/attitude in someone’s voice, and Kornreich et al (2002) confirmed that alcoholics are impaired in emotional processing such as interpreting non-verbal emotional cues and recognizing facial expressions of emotion (Philippot et al 1999; Kringelbach et al 2001; Kornreich et al 2001, 2002; Indersmitten et al 2003). Using computer images and different intensities of morphed facial expressions, Kornreich and co-workers found that alcoholics overestimated the intensity of all emotional expressions, misinterpreted emotional expressions (except fear) at all levels of intensity, and were unaware of their misperceptions (Philippot et al 1999). Oscar-Berman et al (1990) also reported that, compared with nonalcoholic controls, alcoholics judged photographs of facial emotional expressions to be more intense. When listening to sentences, the alcoholics also had some difficulty judging emotional intonations and emotional semantic content.
A decoding deficit for anger and contempt also has been reported. In a recent study by Townshend and Duka (2003), the authors demonstrated in line with previous studies that alcoholics were impaired in their recognition of emotional facial expressions. However, whereas previous studies (Philippot et al 1999; Kornreich et al 2001, 2002) had found that alcoholics overestimated the intensity of each of the emotional facial expressions, Townshend and Duka (2003) showed that alcoholics inappropriately enhanced the intensity of fear in the facial expressions. Additionally, alcoholics showed a different response than the controls in the recognition of anger and disgust. Alcoholics tended to overestimate the amount of the relevant emotion. Such a response could be explained by a tendency in alcoholics to exaggerate emotions and may be related to a disinhibitory effect of long-term alcohol use (Oscar-Berman et al 1990; Philippot et al 1999).
Other considerations in alcoholics’ responses to emotional materials are length of sobriety and drinking history. For example, Kornreich et al (2001) found that recently detoxified alcoholics (three weeks of sobriety) made significantly more errors in identifying emotional facial expressions and overestimated the intensity of facial expressiveness than did either longer term recovered alcoholics (two months or more) or normal controls. Decoding accuracy and intensity evaluations varied with different affects depicted. Expressions of anger and disgust resulted in sustained error judgments. Additionally, Harding et al (1996) noted a positive correlation between the degree of brain atrophy and the rate and amount of alcohol consumed over a lifetime.
To explain emotional dysfunction, theorists have made reference to participation of multiple regions of the brain (Gainotti 2001) (eg, see Table 1 and Figure 1). In alcoholics, there is growing evidence for involvement of many of the regions responsible for emotional functioning (Bowirrat and Oscar-Berman 2005). We begin by examining cortical and white-matter changes, followed by changes in the limbic system and the cerebellum.
Table 1.
Brain structures involved in emotional functions
| Control/inhibition of emotional responses | Orbitofrontal cortex |
| Emotional evaluation | Amygdala |
| Emotional learning and memory | Hippocampus; cerebellum |
| Autonomic component | Hypothalamus |
| Emotional response components | Ventral striatum |
| Expressive motor components: | |
| – Automatic reactions to emotionally provocative stimuli | Right hemisphere |
| – Consciously learned aspects of emotional experience and responses | Left hemisphere |
Figure 1.
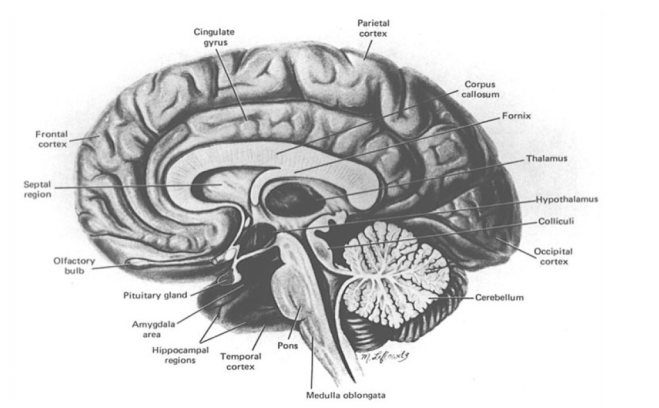
The human brain in cross section. Cortical, limbic, and cerebellar regions are highly vulnerable to alcoholism-related damage. Among the regions discussed in this paper are the frontal lobes, amygdala, hippocampus, and cerebellum. Source: Oscar Berman M. Corsini/Concise Encyclopedia of Psychology. Copyright © (2005 Wiley). Reprinted with permission of John Wiley & Sons, Inc.
Cortical changes
Alcoholism-related changes in emotion, cognition, and behavior have been linked to extensive regions of damage (Dao-Castellana et al 1998; Sullivan, Deshmukh, et al 2000; Kubota et al 2001; Moselhy et al 2001; Ravaglia et al 2002). In vivo MRI studies have shown similar extents of gray matter and subjacent white matter volume deficits (Jernigan et al 1991; Pfefferbaum et al 1992; Fein et al 2002). These patterns differ from neuropathological studies, which are more consistent in reporting white matter than gray matter volume abnormalities (Harper et al 1985, 1988; De la Monte 1988; Pfefferbaum et al 1992; Harper et al 1998). In vivo diffusion tensor imaging, a relatively new imaging modality that is useful for visualizing white matter bundles and microstructure (Basser and Pierpaoli 1996), also has revealed disruption of brain white matter microstructural integrity in alcoholic men (Pfefferbaum et al 2000) and women (Pfefferbaum and Sullivan 2002).
The frontal lobes
The frontal lobes are connected with all of the other lobes of the brain and they receive and send fibers to numerous subcortical structures as well (Fuster 1997). While control of motor function takes place in the posterior region of the frontal lobes, the anterior region of the frontal lobes (prefrontal cortex) plays a kind of executive regulatory role within the brain (Giancola and Moss 1998; Goldberg 2001; Lichter and Cummings 2001). More precisely, prefrontal cortex is important for cognitive flexibility, problem solving, attention, speed in information processing, abstractive and planning skills, inhibition, and suppression of irrelevant information (Raine et al 1998; Blair and Cipolotti 2000; Davidson et al 2000). Normally, the prefrontal cortex inhibits the occurrence of unnecessary or unwanted behaviors, but disruptions of these inhibitory functions often will release previously inhibited behaviors. For example, patients with lesions of the frontal lobes exhibit impulsive aggression (Kuruoglu et al 1996; Deckel 1999; Blair and Cipolotti 2000). Additionally, induction studies of transient anger are associated with orbitofrontal activation, suggesting a role for this region in anger regulation (Dougherty et al 1999; Kimbrell et al 1999). Moreover, as noted in a review by Giancola (1995), and subsequently stressed by Hoaken et al (1998), executive cognitive functions have proved to be involved in the expression of aggressive behavior (Ratti et al 2002).
Prefrontal cortex is not functionally uniform. Major subdivisions are the dorsolateral prefrontal region and the orbitofrontal cortical region (OFC). Dorsolateral prefrontal cortex has reciprocal projections to and from other neocortical association cortices, limbic structures such as the hippocampus (via the cingulate cortex and ventral prefrontal cortex), and diencephalic regions (eg, lateral regions of the dorsomedial thalamic nucleus, as well as to ventral and anterior thalamic areas) (Fuster 1997). Dorsolateral prefrontal cortex has non-reciprocal efferent connections with basal ganglia sites, sending fibers to the anterolateral portion of the head of the caudate nucleus. The dorsolateral prefrontal cortex is a primary neocortical target of ascending dopaminergic innervation; an ascending catecholaminergic pathway originating in the midbrain ventral tegmental area also projects onto dorsolateral prefrontal sites (for reviews, see Oscar-Berman et al 1991; Panksepp 1998). Neuroanatomical connections with OFC parallel those of dorsolateral cortex, but the two systems are distinctly different. Primary OFC connections are with the medial thalamus (the magnocellular region of the dorsomedial nucleus), the hypothalamus, the ventrolateral portion of the head of the caudate, and the amygdala (Fuster 1997). The OFC is host to a variety of neurochemical influences because of its intimate connectivity with hypothalamic and limbic sites, but it appears to be linked more with forebrain cholinergic than with catecholaminergic systems (Oscar-Berman et al 1991). In general, compared with dorsolateral cortex, OFC is more densely interconnected with limbic sites and with the basal forebrain, and less interconnected with other neocortical association areas.
Many studies have found the frontal lobes to be more susceptible to alcohol-related brain damage than other cerebral regions. These results were based on neuro-psychological and neuroradiological data (Ratti et al 1999; Oscar-Berman 2000; Sullivan 2000; Mann et al 2001). In addition, studies of brain pathology at autopsy have revealed decreased neuron density in the frontal cortex of alcoholics (eg, see Kril et al 1997). Kril and collaborators (1997) established that 15%–23% of cortical neurons are selectively lost from the frontal association cortex following chronic alcohol consumption.
Magnetic resonance imaging studies have shown frontal lobe volume losses in alcoholic subjects (Pfefferbaum et al 1997), and prefrontal neurobehavioral dysfunctioning has been frequently observed in alcoholics with and without Korsakoff’s syndrome (Kril et al 1997; Oscar-Berman 2000). Such abnormalities have been identified with reduced regional blood flow measurements (Dally et al 1988; Melgaard et al 1990) and with measurements of lower glucose metabolism throughout the brain (including prefrontal cortex) during alcohol intoxication (Volkow et al 1990).
Frontal lobe blood flow (Nicolas et al 1993) and metabolism (Volkow et al 1992) may decrease in alcoholics before significant shrinkage or major cognitive problems become detectable (Nicolas et al 1993; Wang et al 1993). Cognitive functions and motor coordination may improve at least partially within three to four weeks of abstinence (Oscar-Berman et al 1997; Sullivan et al 2000) accompanied by at least partial reversal of brain shrinkage (Shear et al 1994; Pfefferbaum et al 1995) and some recovery of metabolic functions in the frontal lobes (Johnson-Greene et al 1997) and cerebellum (Martin et al 1995; Seitz et al 1999). Frontal lobe blood flow continues to increase with abstinence, returning to approximately normal levels within four years (Gansler et al 2000). Relapse to drinking leads to resumption of shrinkage (Pfefferbaum et al 1995), continued declines in metabolism and cognitive function (Johnson-Green et al 1997), and evidence of neuronal cell damage (Martin et al 1995).
The right hemisphere
Although the left and the right cerebral hemispheres are complementary in their functions, each half has its own expertise (Pegna et al 2002). The left hemisphere plays a special role in processing information analytically and sequentially, and it is important for communication, logic, and language. The right hemisphere plays a dominant role in emotional functions, creativity, musical abilities, and coordinating interactions with the three dimensional world around us (eg, spatial cognition) (Oscar-Berman and Schendan 2000). However, there is variability in the distribution of brain regions showing lateralization for emotional functions, and a number of studies have failed to find lateralization at all (Mammucari et al 1988; Caltagirone et al 1989; Kowner 1995; Wager 2003).
Nonetheless, evidence from healthy adults and from brain-damaged patients has shown that the right hemisphere is superior to the left in analyzing emotional content in linguistic and non-linguistic communication (Borod et al 1998, 2000; Springer and Deutch 1998). The hypothesis of right hemisphere dominance for emotional functions is supported by a large body of clinical and experimental evidence (Gainotti 1997, 2001) and concerns several components of the emotional behavior. Thus, Ross (1984), Blonder et al (1993), and Borod et al (1997) have repeatedly stressed right hemisphere dominance for non-verbal emotional communication. Heilman et al (1978) and Meadow and Kaplan (1994) have shown that the right hemisphere plays a critical role in autonomic functions, and Mammucari et al (1988) and Wittling and Roschmann (1993) have obtained data showing that the right hemisphere is also critically involved in the subjective experience of emotions.
Right hemisphere dominance is usually very clear for negative emotions, but becomes less clear when some positive emotions are taken into account. To explain the right hemisphere’s differential involvement in positive and negative emotions, some authors have assumed separate hemispheric specializations for these two categories of emotions (Borod 2000; Murphy et al 2003; Davidson et al 2004). In other words, both hemispheres process emotion, but each hemisphere is specialized for particular types of emotion, particularly in the lateral frontal cortex (Wager 2003). In one formulation, the left hemisphere controls positive emotions and the right hemisphere controls negative emotions (Sackeim et al 1978; Robinson and Starkstein 1989; Davidson 1992; Gur et al 1994). A disproportional number of patients who have suffered trauma to the left frontal lobe, especially to the lateral prefrontal cortex or basal ganglia, become depressed (Paradiso et al 1999; Narushima et al 2003). Patients with right frontal damage, however, are more likely to show signs of inappropriate cheerfulness and mania (Starkstein et al 1989). Further, individuals with affective disorders have shown abnormal laterality patterns that may be suggestive of right hemisphere dysfunction (Jaeger et al 1987; Liotti et al 1991; Phillips et al 2003). There also is evidence of possible premorbid bilateral- and right-frontal abnormalities in individuals at risk for alcoholism, a subset of whom display impulsivity, insensitivity to reinforcement, antisocial personality disorder, etc (Tarter et al 1985; Hesselbrock 1991; Pihl and Peterson 1991; Holdcraft et al 1998; Lappalainen et al 1998).
Rather than an opposite dominance of the right hemisphere for negative emotions and the left hemisphere for positive emotions, there simply may be a stronger involvement of the right hemisphere for negative as compared with positive emotions. This asymmetry may be explained by assuming that some positive emotions (eg, happiness) may be better handled by the left hemisphere because these emotions can be used intentionally for functions of approach and of social communication. Whatever the mechanism, it seems that the right hemisphere is more involved than the left in control of negative emotions, and right hemisphere abnormalities contribute to some domains of psychopathology, such as affective disorders (Jaeger et al 1987; Starkstein et al 1987; Liotti et al 1991) and content-specific delusions (Feinberg 1999). Further, there is evidence of possible premorbid bilateral- and right-frontal abnormalities in individuals at risk for alcoholism, a subset of whom display impulsivity, insensitivity to reinforcement, antisocial personality disorder, etc (Tarter et al 1985; Hesselbrock 1991; Pihl and Peterson 1991; Lappalainen et al 1998).
Behavioral studies have shown that in healthy humans, the left side of the face is emotionally more expressive than the right (Sackeim et al 1978). Additionally, processing of positive emotions is potentiated when emotional stimuli are presented to the left hemisphere (via the right ear or the right visual field), and negative emotions are potentiated when presented to the right hemisphere (Davidson et al 1987; Burton and Levy 1989). Emotional intonation (prosody) is more easily recognized when presented to the left ear (Erhan et al 1998), and stimuli presented in the left visual field are judged as more emotional (Levine and Levy 1986) and elicit greater autonomic responses (Spence et al 1996).
As noted earlier, differences between the two cerebral hemispheres can be seen easily in cases of unilateral brain damage (Lezak 1995). Of interest to the present discussion is the fact that alcoholic individuals have difficulty on tasks that resemble those on which patients with damage to the right hemisphere also encounter problems. In particular, patients with right hemisphere lesions, as well as alcoholics, are disproportionately impaired on non-verbal visuospatial tasks, as assessed by Performance IQ subtests (Oscar-Berman 2000). Right hemisphere patients also show emotional abnormalities such as a diminished reaction to catastrophic events (Heilman 1997; Crucian et al 2000). Additionally, deficits in prosody (emotion speech characteristics) have been found in patients with right hemisphere frontal damage (Ross and Mesulam 1979) and in alcoholics (Monot et al 2001), and deficits in recognition of emotional facial expressions have been linked to right hemisphere damage (Weddell 1994; Mandal et al 1996) and to alcoholism (Kornreich et al 2001a, 2001b).
Findings from lesion studies and from neuroimaging studies in healthy subjects suggest that an increase in right-sided activation in various sectors of the prefrontal cortex is associated with increased negative affect. Preliminary results from an fMRI study (Howard et al 2003) have suggested that while nonalcoholic controls exhibited strong right frontal activation when viewing faces (see Figure 2), especially with negative emotional expressions, alcoholics displayed reduced right frontal activation (Howard et al 2003).
Figure 2.
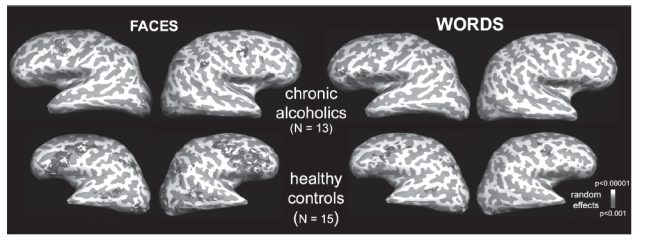
A comparison of fMRI activations observed in chronic alcoholics (N = 13) versus healthy controls (N = 15) during encoding of emotional words and emotional facial expressions. The absence of prefrontal activity of the alcoholics for emotional faces is striking.
Because of a similarity in deficits seen in alcoholics and patients with damage to the right hemisphere, it has been hypothesized that right-brain functions, visuospatial, and emotional functions in particular, are more vulnerable to the effects of alcoholism than are left-brain functions. Studies of people with brain lesions have provided evidence that disruption of the integrity of the corpus callosum can contribute to right hemisphere functional decline as well as diminution of interhemispheric (cross-callosal) transfer ability accompanying bilateral cortical atrophy (impairment in sensory and cognitive integration [Fabri et al 2001] and interhemispheric transmission [Brown et al 2000; Curran et al 2001]). Diffusely distributed bilateral cortical atrophy and thinning of the corpus callosum might be interpreted as a selective right hemisphere functional deficit using conventional neuropsychological tests because (a) right hemisphere functions may have less cortical representation than left hemisphere functions (possibly because left hemisphere functions are used more frequently), or (b) The absence of prefrontal activity of the alcoholics for emotional faces is striking. bilateral damage could cause an interhemispheric collaboration dysfunction (eg, excessive interhemispheric inhibition or a cross-callosal transfer dysfunction), which affects visuospatial and emotional functions more than linguistic functions.
To test these notions, Schulte et al (2004) examined the processing of visual information using redundant targets in alcoholics and healthy controls. Among the authors’ predictions were (a) that interhemispheric parallel processing of information would be compromised in alcoholics relative to controls, (b) interhemispheric transfer time would be prolonged in alcoholics relative to controls, and (c) interhemispheric transfer time prolongation would be greater in older than younger subjects. They observed that the effects of redundant targets were smaller in older alcoholics than in older subjects with or without history of alcoholism suggesting reduced interhemispheric neural summation. Also, the level of performance was associated with callosal size in controls in contrast to alcoholics. A decrease in the size of the corpus callosum was related to prolonged interhemispheric transfer time, and thinning of the corpus callosum occurred in alcoholism and with aging. Others also had reported an interaction between alcoholism and aging for the corpus callosum (Pfefferbaum et al 1996, 2002). There also was an interaction between aging and alcoholism with regard to brain tissue volumes, with volume abnormalities greater in older than younger alcoholics relative to age norms. Finally, in vivo MRI (Pfefferbaum et al 1996) and post-mortem studies (Harper and Kril 1990) have revealed significant callosal thinning in chronic alcoholics. In addition, in vivo studies based on diffusion tensor imaging have shown compromise of callosal fiber coherence in alcoholic men (Pfefferbaum et al 2000) and women (Pfefferbaum et al 2002), the extent of which relates to the degree of attentional and working memory deficits. One might speculate that thinning of the corpus collosum may render alcoholics less able to inhibit negative affect in right hemisphere circuits. Additionally, there is a growing literature on the relationship between handedness and abnormal hemispheric organization in alcoholics (eg, Ellis and Oscar-Berman 1989; Oscar-Berman and Schendan 2000; Sperling et al 2000). For example, Sperling and colleagues, in a study of 250 alcohol-dependent inpatients, found support for the hypothesis of deviant laterality in the presence of an elevated frequency of developmental risk factors. Type II alcoholic personalities (early drinking onset, antisocial personality characteristics, and refractory to treatment; Cloninger 1987) may be the most vulnerable to thinning of the corpus collosum and perhaps even to emotional processing difficulties (Sperling et al 2000).
Another line of research has examined possible cerebral hemispheric asymmetries in the regulation of different neurotransmitter systems. For example, Tucker and Williamson (1984) claimed that there is abundant evidence for greater serotonin regulation of right hemisphere systems and greater catecholaminergic and cholinergic regulation of left hemisphere systems. Perhaps the genetic anomalies noted in previous sections of this paper (Temperament in relation to alcoholism) reduce the regulatory capacity of serotoninergic systems in selected right-hemisphere sites concerned with processing emotions. If so, it would follow that an individual would be more vulnerable to episodes of negative affect and the use of alcohol to cope. Thus, a vicious cycle would be perpetuated.
The limbic system
Primary areas of the limbic system include the hypothalamus, amygdala, hippocampus, septal nuclei, and anterior cingulate gyrus. Functions of the limbic system include monitoring of internal homeostasis, mediating memory and learning, and contributing to emotions. In this section, we discuss the amygdala and hippocampus, because of their essential roles in emotion and alcoholism.
The amygdala
The amygdala is a small almond shaped structure, deep inside the anteroinferior region of the temporal lobe. The amygdala is an extremely heterogeneous brain area consisting of 13 nuclei and cortical regions and their subdivisions (Pitkänen 2000; Sah et al 2003). It connects with prefrontal cortex, the hippocampus, the septal nuclei, and the medial dorsal nucleus of the thalamus. These connections make it possible for the amygdala to play its important role on the mediation and control of major affective states such as love, fear, rage, anxiety, and general negative affectivity (Aggleton 2000; Pitkänen et al 2000; Amaral et al 2003). The amygdala, being a center for the identification of danger, is fundamental for self-preservation.
In humans, facial expressions of negative affect are examples of fearful stimuli. In fact, facial expressions convey such strong emotional information that merely observing anger or fearful faces elicits visceral responses, including increased heart rate and sweating (Ohman and Soares 1998). Neuroimaging studies (Davis and Whalen 2001) have illustrated that these fearful responses to facial expressions are processed and largely mediated by the amygdala (having connections to both early sensory processing areas and autonomic reflex centers). Furthermore, amygdala responses to fearful faces have been observed with neuroimaging scans even in the absence of conscious awareness of their presentation to subjects (Whalen et al 1998).
A number of studies have linked the amygdala to the processing of motivational significance of stimuli and to the control of emotion (Davis 1992; Everitt et al 2003; LeDoux 2000, 2003). These include studies based on single-unit recordings in rats (eg, Schoenbaum et al 2000), studies in monkeys (Rolls 1999), and human fMRI studies (eg, Aharon et al 2001; Breiter et al 2001).
A “startle response” or “startle reflex” is when people jump at the sound of a loud, unexpected noise. The reflex is generally dependent on cues: it can be made stronger by viewing negative photographs, such as traffic accident victims; it can be made weaker by positive photographs, such as happy babies and favorite foods. The amygdala is essential for the changes in the defensive startle reflex. The amygdala is involved in forming emotions in response to exposure to aversive emotional pictures or warning cues that an individual sees and hears (Levenston et al 2000). The amygdala is controlled in part by the brain’s dopamine system, the same system that responds to alcohol and produces feelings of pleasure when good things happen.
Functional neuroimaging studies of emotional processing and inhibitory control have revealed an important modulatory role of the prefrontal cortex, especially in the right hemisphere, on amygdala responses (Nakamura et al 1999; Narumoto et al 2000; Beauregard et al 2001). In line with this evidence, a significant response was observed bilaterally in the amygdala to facial expressions, but the magnitude of the right amygdala response was larger than that of the left. It was the response of the right amygdala alone that habituated over successive scans (Hariri et al 2002), and a larger response was illustrated in the right (not the left) amygdala.
At a functional level, the stimulus-specific laterality of these responses reflects the inherent nature and value of these stimulus types. The right biased response to faces is consistent with studies implicating right hemisphere brain regions in general, and the right amygdala specifically, for processing facial expressions, especially those of negative affect (Ahern et al 1991; Adolphs et al 2001). Furthermore, Hariri et al (2002) found that dextroamphetamine, a nonspecific monoaminergic agonist and anxiogenic, selectively potentiates the response of only the right amygdala during the perceptual processing of angry and fearful faces, suggesting that this structure may be especially critical in processing the emotional content of stimuli. In addition, human subjects who were exposed to erotic stimuli had increased levels of activation of the right amygdala and right temporal pole as measured by fMRI (Beauregard et al 2001). The right amygdala has also been shown to be associated with enhanced recall memory for emotional films (Cahill et al 2000). Finally, recent studies have demonstrated the involvement of the right amygdala in contextual conditioned fear and in stress or emotional related processes (Anderson and Teicher 1999; Baker and Kim 2004; Scicli et al 2004).
The hippocampus
The hippocampus (HP) is a horseshoe shaped sheet of neurons located on the floor of each lateral ventricle within the temporal lobes and adjacent to the amygdala. As part of the limbic system, it is intimately involved in motivation and emotion, and it also plays a central role in the formation of memories (Burgess et al 2002). The HP consists of the complex interfolded layers of the dentate gyrus and cornus ammonis, which are continuous with the subiculum, which, in turn, merges with the parahipocampal gyrus. The anatomy of the HP is closely associated with subcortical structures that contribute to the hypothalamic-pituitary-adrenal axis (Kjelstrup et al 2002). A recent study also demonstrated that encoding of emotional memories depends on the HP in conjunction with the amygdala as well as their interaction with each other (Richardson et al 2004). Although the idea that the HP may play a role in brain mechanisms underlying anxiety is not new (McNaughton and Gray 2000; Deacon et al 2002), there is now mounting evidence that the ventral HP plays an important role in a brain system associated with fear and/or anxiety (Bannerman, Deacon, et al 2002; Bannerman, Grubb, et al 2002; Kjelstrup et al 2002; McHugh et al 2004).
Neuroimaging studies have demonstrated reduction of hippocampal volume in alcoholics compared with control subjects (Agartz et al 1999; Laakso et al 2000; Pfefferbaum and Sullivan 2002; Bleich, Sperling, et al 2003; Bleich, Wilhelm, et al 2003). One MRI study measured volumes of the HP in late-onset alcoholics (Type 1) and violent early-onset alcoholics (Type 2) compared with nonalcoholic controls. The right, but not left, HP was significantly smaller in both alcoholic groups. While there was no correlation between the hippocampal volumes with age in the control subjects, there was tendency towards decreased volumes with aging and also with the duration of alcoholism in the Type 1 alcoholic subjects (Laakso et al 2000). Activity in the right HP decreased the more remote the autobiographical memories; the gradient of this decrease spanned decades. (Eleanor et al 2003). This suggested that the right HP remains active for memories that are 5 or even 10 years old, and likewise for memories 30 years old, albeit to a much lesser degree.
Lateral asymmetry in remoteness may also interact with age. Many individuals with selective bilateral hippocampal damage are older adults (Spiers et al 2001), as are many semantic dementia cases. Older subjects may be more dependent on the right HP than young subjects. Since the right HP is more active for recent events, then damage to this structure in older subjects may be more likely to produce a temporal gradient. Interestingly, Kopelman et al (1989) found that young amnestic patients showed a relatively flat temporal gradient, whereas older amnestic patients demonstrated a more marked gradient.
Chronic alcoholism significantly impairs hippocampal long-term potentiation (Miranda, Nelson et al 2002) and produces progressive learning and memory deficits across a variety of behavioral tests, including active avoidance (Walker et al 1971) and spatial memory (Santin et al 2000). Reduction of hippocampal volume in alcoholics is reversible after short periods of abstinence (White et al 2000). The loss of hippocampal volume has been attributed to changes in white matter (Harding et al 1997), but the incorporation of newly formed neurons to the dentate gyrus could also be affected by alcohol. Similarly, hippocampal-dependent cognitive functions have also shown reversibility after comparable periods of abstinence.
Results of a recent study suggest that the marked effect of ethanol on the survival of newly formed neurons in the adult HP could result in impairment of hippocampal-dependent cognitive functions, or, alternatively, the changes in cognition observed in alcoholism could lead to decreased neuronal survival (Herrera et al 2003). Neurogenesis is primarily a developmental process that involves the proliferation, migration, and differentiation into neurons of primordial stem cells of the central nervous system (Gage 2000). Neurogenesis declines until it ceases in the young adult mammalian brain with two exceptions: the olfactory bulb and the hippocampus produce new neurons throughout adult life. However, multiple factors seem to regulate adult neurogenesis including hormones, neurotransmitters, and trophic factors (Gage 2000).
The ethanol-induced reductions in hippocampal neurogenesis can be attributed to two general mechanisms: an effect on cell proliferation or on cell survival. These changes in the hippocampal structure could be part of the anatomical basis for the cognitive deficits described in alcoholism. The hippocampus is a target site for the teratogenic effects of ethanol (West and Pierce 1986). Morphological changes in this brain region may play a critical role in the manifestation of mental deficiency and behavioral abnormalities in individuals with fetal alcohol syndrome or alcohol-related neurodevelopmental disorder (Institute of Medicine 1996). There is evidence that certain hippocampal neuronal cell types are particularly sensitive to ethanol teratogenicity. Chronic exposure of the developing HP to ethanol can result in selective damage, such as a decrease in the number of CA1 pyramidal cells in the adult pig (Abdollah et al 1993; Gibson et al 2000) and rat (Bonthius and West 1990; Miller 1995). One study of human alcoholics aged 45 years and under, reported an early neuronal loss of the dentate gyrus and the ammonic fields CA1 through CA4 (Bengochea and Gonzalo 1990), and another study found glial cell loss (especially astrocytes and oligodendrocytes) in the hippocampus of alcoholics (Korbo 1999).
The cerebellum
The cerebellum is a portion of the brain that coordinates movement of voluntary muscles, balance, and eye movements. It contains about half of the brain’s neurons, but these particular nerve cells are so small that the cerebellum accounts for only 10% of the brain’s total weight. The cerebellum consists mainly of two large tightly folded lobes joined at the middle by the vermis. Also located anteriorly are the small flocculonodular lobes (flocculi). The cerebellum connects with the other brain structures through the cerebellar peduncles, located to the anterior of the cerebellum. Deep within the cerebellum is white matter within which lie the deep cerebellar nuclei. Of these, the cerebellar dentate nucleus is the most recognizable. Five different nerve cell types make up the cerebellum: stellate, basket, Purkinje, Golgi, and granule cells. The Purkinje cells are the only ones to send axons out of the cerebellum.
Atrophy of the cerebellum is commonly associated with alcoholism. Gross vermian atrophy is commonly seen postmortem in alcoholics (Phillips et al 1987), and it also has been observed with in vivo neuroimaging techniques (Sullivan et al 2000). White matter volume of the cerebellar vermis is significantly reduced (Baker et al 1999; Pentney et al 2002; Sullivan et al 2003), and cerebellar vermian atrophy occurs in 25%–40% of all alcoholics. Vermal white matter volume was reduced in ataxic alcoholics by 42%. It occurs even more often in people with additional thiamine deficiency, with 35%–50% of those individuals showing evidence of superior vermian degeneration (Victor et al 1989).
Alcoholics with Wernicke-Korsakoff’s syndrome have shown a significant decrease in Purkinje cell density in the cerebellar vermis and molecular layer volume (Baker et al 1999). A significant reduction in Purkinje cell numbers in the flocculi suggests disruption of vestibulocerebellar pathways. This is of particular interest given recent data showing the importance of the cerebellum in the organization of higher order cerebral functions (Schmahmann and Sherman 1998; Schmahmann 2000; Sullivan et al 2003).
A number of functions have been attributed to the cerebellum, and there is growing evidence for its role in certain aspects of learning and memory (Buckner et al 1996). Schneider and colleagues (2001) proposed that cue-induced craving, in this case alcohol-induced craving, may involve conditioned emotional reactions that are mediated by the amygdala, as well as learned memory associations that are mediated by the cerebellum. Alternatively, stimulation may have activated cerebellar functions involved in motor or multisensory coordination. In their study, the authors demonstrated that the amygdalar and cerebellar activations observed before therapy in abstinent alcoholic patients may represent aspects of emotion, motivation, and memory in cue-induced craving (Schneider et al 2001).
In addition to interactions with limbic system structures, the cerebellum also influences functions classically associated with frontal lobe functioning, suggesting a role for frontocerebellar circuitry (Schmahmann 1997, 2004). As noted earlier in the section on frontal lobes, this part of the brain has executive control functions such as cognitive flexibility, aspects of attention, speed in information processing, inhibition of preservative errors, abstractive and planning skills. There is ample evidence for alcohol’s untoward effects on the structure and function of the cerebellum and frontal lobes, and disruption of this circuitry is a potential mechanism underlying behavioral impairment characteristic of alcoholism (for reviews, see Oscar-Berman 2000; Ilinsky and Kultas-Ilinsky 2002; Sullivan 2003; Sullivan et al 2003).
Genetic influences on brain development
Of special interest is a growing body of evidence that genetic anomalies impact some of the same brain areas that are vulnerable to the effects of alcoholism, and those that are involved in emotional processing. For example, Yamasaki et al (2003) found that the human UBE3A gene (which shows brain-specific partial imprinting, ie, it has an alteration in chromatin affecting its expression but not its DNA sequence) was expressed predominantly in the limbic system (including the hippocampus), in cerebellar Purkinje cells, and in the olfactory bulbs. (Imprinting is a reversible form of gene inactivation but is not a mutation.) With the possible exception of the olfactory bulbs, all of the regions reported by Yamasaki et al (2003) to be affected by UBE3A are known to be affected by chronic alcoholism. As we noted earlier in the section, Genetics of temperament in relation to alcoholism, Nurnberger et al (2002) found evidence for antisocial alcoholism at quantitative trait locus (QTL) 11p. The subtelomeric region of 11p (11p15.5) contains three genes: IGF2 (a growth factor), INS (insulin), and TH (tyrosine hydroxylase) that lie in an interval of less than 50 kb. In addition, this region contains genes (eg, KCNQ1OT1) that code for the M-current and similar potassium channel mediated effects. Finally, two genes important for catecholaminergic expression in the CNS are localized to the 11p15.5 region: TH and the gene that encodes the dopamine receptor D4 (DRD4). Interestingly, many of the genes in area 11p15 are imprinted. These observations are potentially important for alcoholism research because of the role of genetic influences in the etiology of neurological and neurobehavioral abnormalities. Perhaps the same genes involved in brain anomalies are also involved in alcoholism. However, as noted by Dick and Foroud (2003), sequencing of the human genome will make the cataloging of human genes and genetic variation available to researchers who will advance the association of candidate genes with alcoholism. Once replicable associations are established, it will still remain a challenge to identify the causative genetic variants responsible for the role of that gene in alcohol dependence.
Summary
Genetic studies of personality traits have provided evidence that temperament dimensions are associated with distinct neurochemical substrates contributing to specific personality phenotypes, and that certain aspects of abnormal emotional traits in alcoholics may be inherited. Additionally, an individual’s genetic history can impact both a tendency toward alcoholism and the development of anomalies in areas of the brain involved in emotional processing, most notably the cerebral cortex, limbic system structures, and the cerebellum. Results of in vivo MRI and post-mortem neuropathological studies of alcoholics indicate that the greatest cortical loss occurs in the frontal lobes, with concurrent thinning of the corpus callosum. Additional damage has been documented for the amygdala and hippocampus, as well as in the white matter of the cerebellar vermis. All of the critical areas of alcoholism-related brain damage are important for normal emotional functioning. When changes occur in these brain regions as a consequence of genetic aberrations or chronic ethanol use, or both, corresponding changes in emotional functions are to be expected. Such changes have been observed in the perception and evaluation of emotional facial expressions, interpretation of emotional intonations in vocal utterances, and appreciation of the meaning of emotional materials.
Acknowledgments
The writing of this paper was supported by funds from the US Department of Health and Human Services, NIAAA (R37-AA07112 and K05-AA00219), and by funds from the Medical Research Service of the US Department of Veterans Affairs. We thank an anonymous reviewer for helpful suggestions.
References
- Abdollah S, Catlin MC, Brien JF. Ethanol neuro-behavioural teratogenesis in the guinea pig: behavioural dysfunction and hippocampal morphological change. Can J Physiol Pharmacol. 1993;71:776–82. doi: 10.1139/y93-116. [DOI] [PubMed] [Google Scholar]
- Adamson MD, Kennedy J, Petronis A, et al. DRD2 dopamine receptor genotype and CSF monoamine metabolites in Finnish alcoholics and controls. Am J Med Genet. 1995;60:199–205. doi: 10.1002/ajmg.1320600306. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Damasio H, Tranel D, et al. Cortical systems for the recognition of emotion in facial expressions. J Neurosci. 1996;16:7678–87. doi: 10.1523/JNEUROSCI.16-23-07678.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Jansari A, Tranel D. Hemispheric perception of emotional valence from facial expressions. Neuropsychology. 2001;15:516–24. [PubMed] [Google Scholar]
- Agartz I, Momenam R, Rawlings RR, et al. Hippocampal volume in patients with alcohol dependence. Arch Gen Psychiatry. 1999;56:356–63. doi: 10.1001/archpsyc.56.4.356. [DOI] [PubMed] [Google Scholar]
- Aggleton JP. The amygdala: a functional analysis. 2. Oxford: Oxford Univ Pr; 2000. [Google Scholar]
- Aharon I, Etcoff N, Ariely D, et al. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron. 2001;32:537–51. doi: 10.1016/s0896-6273(01)00491-3. [DOI] [PubMed] [Google Scholar]
- Ahern GL, Schomer DL, Kleefield J, et al. Right hemisphere advantage for evaluation emotional facial expressions. Cortex. 1991;27:193–202. doi: 10.1016/s0010-9452(13)80123-2. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Bauman MD, Capitanio JP, et al. The amygdala: is it an essential component of the neural network for social cognition? Neuropsychologia. 2003;41:517–22. doi: 10.1016/s0028-3932(02)00310-x. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Serotonin laterality in amygdala predicts performance in the elevated plus maze in rats. NeuroReport. 1999;10:3497–500. doi: 10.1097/00001756-199911260-00006. [DOI] [PubMed] [Google Scholar]
- Artigas F. Pindolol, 5 hydroxytryptamine, and antidepressant augmentation. Arch Gen Psychiatry. 1995;52:969–71. doi: 10.1001/archpsyc.1995.03950230083012. [DOI] [PubMed] [Google Scholar]
- Baker K, Harding A, Halliday G, et al. Neuronal loss in functional zones of the cerebellum of chronic alcoholics with and without Wernicke’s encephalopathy. Neuroscience. 1999;91:429–38. doi: 10.1016/s0306-4522(98)90664-9. [DOI] [PubMed] [Google Scholar]
- Baker KB, Kim JJ. Amygdalar lateralization in fear conditioning: evidence for greater involvement of the right amygdala. Behav Neurosci. 2004;118:15–23. doi: 10.1037/0735-7044.118.1.15. [DOI] [PubMed] [Google Scholar]
- Ball S, Tennen H, Kranzler H. Factor replicability and validity of the Temperament and Character Inventory in substance-dependent patients. Psychol Assess. 1999;11:514–24. [Google Scholar]
- Bannerman DM, Deacon RMJ, Offen S, et al. A double dissociation of function within the hippocampus: spatial memory and hyponeophagia. Behav Neurosci. 2002;116:884–901. doi: 10.1037//0735-7044.116.5.884. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Grubb M, Deacon RMJ, et al. Ventral hippocampal lesions affect anxiety but not spatial learning. Behav Brain Res. 2002;139:197–213. doi: 10.1016/s0166-4328(02)00268-1. [DOI] [PubMed] [Google Scholar]
- Barr LC, Goodman WK, McDougle CJ, et al. Tryptophan depletion in patients with obsessive-compulsive disorder who respond to serotonin reuptake inhibitors. Arch Gen Psychiatry. 1994;51:309–17. doi: 10.1001/archpsyc.1994.03950040053007. [DOI] [PubMed] [Google Scholar]
- Basser J, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative diffusion tensor MRI. J Magn Reson. 1996;111:209–19. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. J Neurosci. 2001;21:RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengochea O, Gonzalo LM. Effect of chronic alcoholism on the human hippocampus. Histol Histopathol. 1990;5:349–57. [PubMed] [Google Scholar]
- Benjamin J, Li L, Patterson C, et al. Population and familial association between the D4 dopamine receptor gene and measures of novelty seeking. Nat Genet. 1996;12:81–4. doi: 10.1038/ng0196-81. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Saccone NL, Rice JP, et al. Defining alcohol-related phenotypes in humans. The collaborative study on the genetics of alcoholism. Alcohol Res Health. 2002;26:208–13. [PMC free article] [PubMed] [Google Scholar]
- Blair RJ, Cipolotti L. Impaired social response reversal. A case of ‘acquired sociopathy’. Brain. 2000;123:1122–41. doi: 10.1093/brain/123.6.1122. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Morris JS, Frith CD, et al. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999;122:883–93. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- Bleich S, Sperling W, Degner D, et al. Lack of association between hippocampal volume reduction and first-onset alcohol withdrawal seizure. A volumetric MRI study. Alcohol Alcohol. 2003;38:40–4. doi: 10.1093/alcalc/agg017. [DOI] [PubMed] [Google Scholar]
- Bleich S, Wilhelm J, Graesel E, et al. Apolipoprotein E 4 is associated with hippocampal volume reduction in females with alcoholism. J Neural Transm. 2003;110:401–11. doi: 10.1007/s00702-002-0789-1. [DOI] [PubMed] [Google Scholar]
- Blonder LX, Bowers D, Heilman KM. The role of the right hemisphere in emotional communication. Brain. 1991;114:1115–27. doi: 10.1093/brain/114.3.1115. [DOI] [PubMed] [Google Scholar]
- Blonder LX, Burns A, Bowers D. Right hemisphere facial expressivity during natural conversation. Brain Cogn. 1993;21:44–56. doi: 10.1006/brcg.1993.1003. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, West JR. Alcohol-induced neuronal loss in developing rats: increased brain damage with binge exposure. Alcohol Clin Exp Res. 1990;14:107–18. doi: 10.1111/j.1530-0277.1990.tb00455.x. [DOI] [PubMed] [Google Scholar]
- Borod JC, editor. The neuropsychology of emotion. New York: Oxford Univ Pr; 2000. [Google Scholar]
- Borod JC, Bloom R, Haywood CS. Verbal aspects of emotional communication. In: Beeman M, Chiarello C, editors. Right hemisphere language comprehension: perspectives from cognitive neurosciences. Mahwah: Lawrence Erlbaum Associates; 1998. [Google Scholar]
- Borod JC, Haywood CS, Koff E. Neuropsychological aspects of facial asymmetry during emotional expression: a review of the normal adult literature. Neuropsychol Rev. 1997;7:41–60. doi: 10.1007/BF02876972. [DOI] [PubMed] [Google Scholar]
- Borod JC, Rorie KD, Pick LH, et al. Verbal pragmatics following unilateral stroke: emotional content and valence. Neuropsychology. 2000;4:112–24. doi: 10.1037//0894-4105.14.1.112. [DOI] [PubMed] [Google Scholar]
- Bouchard T. Genes environment, and personality. Science. 1994;61:1700–1. doi: 10.1126/science.8209250. [DOI] [PubMed] [Google Scholar]
- Bowirrat A, Oscar-Berman M. Relationship between dopaminergic neurotransmission, alcoholism, and reward deficiency syndrome. Am J Med Genet Part B (Neuropsychiatr Genet) 2005;132:29–37. doi: 10.1002/ajmg.b.30080. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Aharon I, Kahneman D, et al. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30:619–39. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- Brown LN, Sainsbury RS. Hemispheric equivalence and age-related differences in judgments of simultaneity to somatosensory stimuli. J Clin Exp Neuropsych. 2000;22:587–98. doi: 10.1076/1380-3395(200010)22:5;1-9;FT587. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Raichle ME, Miezin FM, et al. Functional anatomic studies of memory retrieval for auditory words and visual pictures. J Neurosci. 1996;16:6219–35. doi: 10.1523/JNEUROSCI.16-19-06219.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–41. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Burton LA, Levy J. Sex differences in the lateralized processing of facial emotion. Brain Cogn. 1989;11:210–28. doi: 10.1016/0278-2626(89)90018-3. [DOI] [PubMed] [Google Scholar]
- Cahill L. Sex-related influences on the neurobiology of emotionally influenced memory. Ann N Y Acad Sci. 2003;985:163–73. doi: 10.1111/j.1749-6632.2003.tb07080.x. [DOI] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, White NS, et al. Sex-related difference in amygdala activity during emotional influenced memory storage. Neurobiol Learn Mem. 2000;75:1–9. doi: 10.1006/nlme.2000.3999. [DOI] [PubMed] [Google Scholar]
- Caltagirone C, Ekman P, Friesen W, et al. Posed emotional expression in unilateral brain damage patients. Cortex. 1989;25:653–63. doi: 10.1016/s0010-9452(89)80025-5. [DOI] [PubMed] [Google Scholar]
- Cloninger CR. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236:410–16. doi: 10.1126/science.2882604. [DOI] [PubMed] [Google Scholar]
- Cloninger C, Adolfsson R, Svrakic D. Mapping genes for human personality. Nat Genet. 1996;12:3–4. doi: 10.1038/ng0196-3. [DOI] [PubMed] [Google Scholar]
- Cloninger C, Przybeck T, Svrakic D, et al. The Temperament and Character Inventory (TCI): a guide to its development and use. St Louis: Washington University, Center for Psychobiology of Personality; 1994. [Google Scholar]
- Cloninger CR, Svrakic DM, Przybeck TR. A psychobiological model of temperament and character. Arch Gen Psychiatry. 1993;50:975–90. doi: 10.1001/archpsyc.1993.01820240059008. [DOI] [PubMed] [Google Scholar]
- Crucian GP, Hughes JD, Barrett AM, et al. Emotional and physiological responses to false feedback. Cortex. 2000;36:623–47. doi: 10.1016/s0010-9452(08)70542-2. [DOI] [PubMed] [Google Scholar]
- Curran T, Hills A, Patterson MB, et al. Effects of aging on visuospatial attention: an ERP study. Neuropsychologia. 2001;39:288–301. doi: 10.1016/s0028-3932(00)00112-3. [DOI] [PubMed] [Google Scholar]
- Dally S, Luft A, Ponsin JC, et al. Abnormal pattern of cerebral blood flow distribution in young alcohol addicts. Br J Addict. 1988;83:105–9. doi: 10.1111/j.1360-0443.1988.tb00458.x. [DOI] [PubMed] [Google Scholar]
- Dao-Castellana MH, Sampson Y, Legault F. Frontal dysfunction in neurologically normal chronic alcoholic subjects: metabolic and neuropsychological findings. Psychol Med. 1998;28:1039–48. doi: 10.1017/s0033291798006849. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anterior electrophysiological asymmetries, emotion, and depression: conceptual and methodological conundrums. Psychophysiology. 1988;35:607–14. doi: 10.1017/s0048577298000134. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anterior cerebral asymmetry and the nature of emotion. Brain Cogn. 1992;20:125–51. doi: 10.1016/0278-2626(92)90065-t. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Jackson DC, Kalin NH. Emotion, plasticity, context and regulation: perspectives from affective neuroscience. Psychol Bull. 2000;126:890–909. doi: 10.1037/0033-2909.126.6.890. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Mednick D, Moss E. Ratings of emotion in faces are influenced by the visual field to which stimuli are presented. Brain Cogn. 1987;6:403–11. doi: 10.1016/0278-2626(87)90136-9. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Shackman AJ, Maxwell JS. Asymmetries in face and brain related to emotion. Trends Cogn Sci. 2004;8:389–91. doi: 10.1016/j.tics.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Davis M, Rainnie D, Cassell M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci. 1992;17:353–75. doi: 10.1016/0166-2236(94)90106-6. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Deacon RMJ, Bannerman DM, Rawlins JNP. Anxiolytic effects of cytotoxic hippocampal lesions in rats. Behav Neurosci. 2002;116:494–7. doi: 10.1037//0735-7044.116.3.494. [DOI] [PubMed] [Google Scholar]
- de Almeida RM, Rowlett JK, Cook JM, et al. GABAA/alpha 1 receptor agonists and antagonists: effects on species-typical and heightened aggressive behavior after alcohol self-administration in mice. Psychopharmacol (Berl) 2004;172:255–63. doi: 10.1007/s00213-003-1661-1. [DOI] [PubMed] [Google Scholar]
- Deckel AW. Tests of executive functioning predict scores on the Mac Andrew Alcoholism scale. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:209–23. doi: 10.1016/s0278-5846(98)00108-0. [DOI] [PubMed] [Google Scholar]
- De la Monte SM. Disproportionate atrophy of cerebral white matter in chronic alcoholics. Arch Neurol. 1988;45:990–2. doi: 10.1001/archneur.1988.00520330076013. [DOI] [PubMed] [Google Scholar]
- Dick DM, Foroud T. Candidate genes for alcohol dependence: a review of genetic evidence from human studies. Alcohol Clin Exp Res. 2003;27:868–79. doi: 10.1097/01.ALC.0000065436.24221.63. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, et al. Direct recording of the output of the motor cortex produced by transcranial magnetic stimulation in a patient with cerebral cortex atrophy. Clin Neurophysiol. 2004;115:112–15. doi: 10.1016/s1388-2457(03)00320-1. [DOI] [PubMed] [Google Scholar]
- Dougherty DD, Shin LM, Alpert NM, et al. Anger in healthy men: a PET study using script-driven imagery. Biol Psychiatry. 1999;46:466–72. doi: 10.1016/s0006-3223(99)00063-3. [DOI] [PubMed] [Google Scholar]
- Ebstein R, Gritsenko I, Nemanov L, et al. No association between the serotonin transporter gene regulatory region polymorphism and the Tridimensional Personality Questionnaire (TPQ) temperament of harm avoidance. Mol Psychiatry. 1997;2:224–6. doi: 10.1038/sj.mp.4000275. [DOI] [PubMed] [Google Scholar]
- Ebstein R, Nemanov L, Klotz I, et al. Additional evidence for an association between the dopamine D4 receptor (D4DR) exon III repeat polymorphism and the human personality trait of novelty seeking. Mol Psychiatry. 1997;2:472–7. doi: 10.1038/sj.mp.4000333. [DOI] [PubMed] [Google Scholar]
- Ebstein R, Novick O, Umansky R, et al. Dopamine D4 receptor (D4DR) exon III polymorphism associated with the human personality trait of novelty seeking. Nat Genet. 1996;12:78–80. doi: 10.1038/ng0196-78. [DOI] [PubMed] [Google Scholar]
- Ekelund J, Lichtermann D, Järvelin MR, et al. Association between novelty seeking and the type 4 dopamine receptor gene in a large Finnish cohort sample. Am J Psychiatry. 1999;156:1453–5. doi: 10.1176/ajp.156.9.1453. [DOI] [PubMed] [Google Scholar]
- Eleanor A, Maguire, Christopher D. Lateral asymmetry in the hippocampal response to the remoteness of autobiographical memories. J Neurosci. 2003;23:5302–7. doi: 10.1523/JNEUROSCI.23-12-05302.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ, Oscar-Berman M. Alcoholism, aging, and functional cerebral asymmetries. Psychol Bull. 1989;106:128–47. doi: 10.1037/0033-2909.106.1.128. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Goldman D. The genetics of alcoholism and alcohol abuse. Curr Psychiatry Rep. 2001;3:144–51. doi: 10.1007/s11920-001-0012-3. [DOI] [PubMed] [Google Scholar]
- Ensor T, Godfrey C. Modeling the interactions between alcohol, crime and the criminal justice system. Addiction. 1993;88:477–87. doi: 10.1111/j.1360-0443.1993.tb02054.x. [DOI] [PubMed] [Google Scholar]
- Erhan H, Borod JC, Tenke CE, et al. Identification of emotion in a dichotic listening task: event-related brain potential and behavioral findings. Brain Cogn. 1998;37:286–307. doi: 10.1006/brcg.1998.0984. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Cardinal RN, Parkinson JA, et al. Appetitive behavior: impact of amygdala-dependent mechanisms of emotional learning. Ann NY Acad Sci. 2003;985:233–50. [PubMed] [Google Scholar]
- Fabri M, Polonara G, Del Pesce M, et al. Posterior corpus callosum and interhemispheric transfer of somatosensory information: an fMRI and neuropsychological study of a partially callosotomized patient. J Cogn Neurosci. 2001;15:1071–9. doi: 10.1162/089892901753294365. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Cardenas V, et al. Cortical gray matter loss in treatment-native alcohol dependent individuals. Alcohol Clin Exp Res. 2002;26:558–64. [PMC free article] [PubMed] [Google Scholar]
- Feinberg TE, Eaton LA, Roane DM, et al. Multiple fregoli delusions after traumatic brain injury. Cortex. 1999;35:373–87. doi: 10.1016/s0010-9452(08)70806-2. [DOI] [PubMed] [Google Scholar]
- Foroud T, Li TK. Genetics of alcoholism: a review of recent studies in human and animal models. Am J Addict. 1999;8:261–78. doi: 10.1080/105504999305677. [DOI] [PubMed] [Google Scholar]
- Freemantle N, Gill P, Godfrey C, et al. Brief interventions and alcohol use. Qual Health Care. 1993;2:267–73. doi: 10.1136/qshc.2.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex: anatomy, physiology, and neuropsychology of the frontal lobes. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
- Gaffney LR, Thorpe K, Young R, et al. Facial skills, expectancies and drinking in adolescents. Addict Behav. 1998;23:587–99. doi: 10.1016/s0306-4603(98)00025-2. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–8. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Emotional disorders in relation to unilateral brain damage. In: Gainotti G, editor; Feinberg TE, Farah M, editors. Behavioral neurology and neuropsychology. New York: McGraw-Hill; 1997. [Google Scholar]
- Gainotti G. Disorders of emotional behaviour. J Neurol. 2001;248:743–9. doi: 10.1007/s004150170088. [DOI] [PubMed] [Google Scholar]
- Galanter M. Alcohol and violence: epidemiology, neurobiology, psychology, family issue. New York: Plenum Pr; 1997. Recent developments in alcoholism. [Google Scholar]
- Gandour J, Wong D, Dzemidzic M, et al. A cross-linguistic fMRI study of perception of intonation and emotion in Chinese. Hum Brain Mapp. 2003;18:149–57. doi: 10.1002/hbm.10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gansler DA, Harris GJ, Oscar-Berman M. Hypoperfusion of inferior frontal brain regions in abstinent alcoholics: a pilot SPECT study. J Stud Alcohol. 2000;61:32–7. doi: 10.15288/jsa.2000.61.32. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Kranzler H, Coccaro E, et al. D4 dopamine receptor (DRD4) alleles and novelty seeking in substance-dependent, personality-disorder, and control subjects. Am J Hum Genet. 1997;61:1144–52. doi: 10.1086/301595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelernter J, Kranzler H, Coccaro E, et al. Serotonin transporter protein gene polymorphism and personality measures in African American and European American subjects. Am J Psychiatry. 1998;155:1332–8. doi: 10.1176/ajp.155.10.1332. [DOI] [PubMed] [Google Scholar]
- George MS, Anton RF, Bloomer C, et al. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Arch Gen Psychiatry. 2001;58:345–52. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- Giancola PR. Evidence for dorsolateral and orbital prefrontal cortical involvement in the expression of aggressive behaviour. Aggress Behav. 1995;21:431–50. [Google Scholar]
- Giancola PR, Moss HB. Executive cognitive functioning in alcohol use disorders. Recent Dev Alcohol. 1998;14:227–51. doi: 10.1007/0-306-47148-5_10. [DOI] [PubMed] [Google Scholar]
- Giancola PR, White HR, Berman ME, et al. Diverse research on alcohol and aggression in humans: In memory of John A Carpenter. Alcohol Clin Exp Res. 2003;27:198–208. doi: 10.1097/01.ALC.0000051024.18538.F0. [DOI] [PubMed] [Google Scholar]
- Gibson MAS, Butters NS, Reynolds NJ, et al. Effects of chronic prenatal ethanol exposure on locomotor activity, and hippocampal weight, neurons, and nitric oxide synthase activity of the young postnatal guinea pig. Neurotoxicol Teratol. 2000;22:183–92. doi: 10.1016/s0892-0362(99)00074-4. [DOI] [PubMed] [Google Scholar]
- Goldberg E. Frontal lobes and the civilized mind. New York: Oxford Univ Pr; 2001. The executive brain. [Google Scholar]
- Goldman D. High anxiety [editorial] Science. 1996;274:1483. doi: 10.1126/science.274.5292.1483. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. The neuropsychology of anxiety. 2. Oxford: Oxford Univ Pr; 2002. [Google Scholar]
- Grobin AC, Matthews DB, Devaud LL, et al. The role of GABA A receptors in the acute and chronic effects of ethanol. Psychopharmacol (Berl) 1998;139(1–2):2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- Gur RC, Skolnick BE, Gur RE. Effects of emotional discrimination tasks on cerebral blood flow: regional activation and its relation to performance. Brain Cogn. 1994;25:271–86. doi: 10.1006/brcg.1994.1036. [DOI] [PubMed] [Google Scholar]
- Harding AJ, Halliday GM, Ng JLF, et al. Loss of vasopressin-immunoreactive neurons in alcoholics is dose-related and time-dependent. Neuroscience. 1996;72:699–708. doi: 10.1016/0306-4522(95)00577-3. [DOI] [PubMed] [Google Scholar]
- Harding AJ, Wong A, Svoboda M, et al. Chronic alcohol consumption does not cause hippocampal neuron loss in humans. Hippocampus. 1997;7:78–87. doi: 10.1002/(SICI)1098-1063(1997)7:1<78::AID-HIPO8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Harper C. The neuropathology of alcohol-specific brain damage or does alcohol damage the brain. J Neuropath Exp Neurol. 1998;57:101–10. doi: 10.1097/00005072-199802000-00001. [DOI] [PubMed] [Google Scholar]
- Harper CG, Kril JJ. Neuropathology of alcoholism. Alcohol Alcohol. 1990;25:207–16. doi: 10.1093/oxfordjournals.alcalc.a044994. [DOI] [PubMed] [Google Scholar]
- Harper C, Kril J, Daly J. Does a moderate alcohol intake damage the brain? J Neurol Neurosurg Psychiatry. 1988;51:909–13. doi: 10.1136/jnnp.51.7.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper CG, Kril JJ, Holloway RL. Brain shrinkage in chronic alcoholics: a pathological study. Br Med J. 1985;290:501–4. doi: 10.1136/bmj.290.6467.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper CG, Kril JJ, Sheedy D, et al. Neuropathological studies: the relationship between alcohol and aging. In: Gomberg ESL, Hegedus AM, Zucker RA, editors. Alcohol problems and aging. Volume 33. Bethesda: NIAAA; 1998. pp. 117–34. [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, et al. The amygdala response to emotional stimuli: a comparison of faces and scenes. NeuroImage. 2002;17:317–23. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Heilman KM. The neurobiology of emotional experience. J Neuropsychiat Clin Neurosci. 1997;9:439–48. doi: 10.1176/jnp.9.3.439. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Schwartz HD, Watson RT. Hypoarousal in patients with neglect and emotional indifference. Neurology. 1978;28:229–32. doi: 10.1212/wnl.28.3.229. [DOI] [PubMed] [Google Scholar]
- Heinz A, Jones DW, Mazzanti C. A relationship between serotonin transporter genotype and in vivo protein expression and alcohol neurotoxicity. Biol Psychiatry. 2000;47:643–9. doi: 10.1016/s0006-3223(99)00171-7. [DOI] [PubMed] [Google Scholar]
- Heinz A, Mann K, Weinberger DR, et al. Serotonergic dysfunction, negative mood states, and response to alcohol. Alcohol Clin Exp Res. 2001;25:487–95. [PubMed] [Google Scholar]
- Herbst JH, Zonderman AB, McCrae RR, et al. Do the dimensions of the temperament and character inventory map a simple genetic architecture? Evidence from molecular genetics and factor analysis. Am J Psychiatry. 2000;157:1285–90. doi: 10.1176/appi.ajp.157.8.1285. [DOI] [PubMed] [Google Scholar]
- Herrera DG, Yague AG, Johnsen-Soriano S, et al. Selective impairment of hippocampal neurogenesis by chronic alcoholism: protective effects of an antioxidant. Proc Natl Acad Sci U S A. 2003;100:7919–24. doi: 10.1073/pnas.1230907100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselbrock MN. Gender comparison of antisocial personality disorder and depression in alcoholism. J Subst Abuse. 1991;3:205–19. doi: 10.1016/s0899-3289(05)80037-9. [DOI] [PubMed] [Google Scholar]
- Hoaken PNS, Giancola PR, Pihl RO, et al. Executive cognitive functions as mediators of alcohol-related aggression. Alcohol Alcohol. 1998;33:47–54. doi: 10.1093/oxfordjournals.alcalc.a008347. [DOI] [PubMed] [Google Scholar]
- Holdcraft LC, Iacono WG, McGue MK. Antisocial personality disorder and depression in relation to alcoholism: a community-based sample. J Stud Alcohol. 1998;59:222–6. doi: 10.15288/jsa.1998.59.222. [DOI] [PubMed] [Google Scholar]
- Howard JA, Oscar-Berman M, Marinkovic K, et al. Washington: Society for Neuroscience; 2003. Affective and cognitive changes in alcoholism: recognition of faces and words. Program nr 419. 15. Abstract Viewer/Itinerary Planner. [Google Scholar]
- Ilinsky IA, Kultas-Ilinsky K. Motor thalamic circuits in primates with emphasis on the area targeted in treatment of movement disorders. Mov Disord. 2002;17:S9–14. doi: 10.1002/mds.10137. [DOI] [PubMed] [Google Scholar]
- Indersmitten T, Gur RC. Emotion processing in chimeric faces: hemispheric asymmetries in expression and recognition of emotions. J Neurosci. 2003;23:3820–5. doi: 10.1523/JNEUROSCI.23-09-03820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton K, Howe C, Battaglia F, editors. Institute of Medicine (US) Fetal alcohol syndrome: diagnosis, epidemiology, prevention and treatment. Washington: National Acad Pr (NAP); 1996. [Google Scholar]
- Jacob T, Waterman B, Heath A, et al. Genetic and environmental effects on offspring alcoholism: new insight using an offspring-of-twins design. Arch Gen Psychiatry. 2003;60:1265–72. doi: 10.1001/archpsyc.60.12.1265. [DOI] [PubMed] [Google Scholar]
- Jaeger J, Borod JC, Peselow E. Depressed patients have atypical hemispace biases in the perception of emotional chimeric faces. J Abnorm Psychol. 1987;96:321–4. doi: 10.1037//0021-843x.96.4.321. [DOI] [PubMed] [Google Scholar]
- Jang KL, Livesley WJ, Vernon PA. Heritability of the big five dimensions and their facets: a twin study. J Pers. 1996;64:575–91. doi: 10.1111/j.1467-6494.1996.tb00522.x. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Butters N, DiTraglia G, et al. Reduced cerebral grey matter observed in alcoholics using magnetic resonance imaging. Alcohol Clin Exp Res. 1991;15:418–27. doi: 10.1111/j.1530-0277.1991.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Johnson-Greene D, Adams KM, Gilman S. Effects of abstinence and relapse upon neuropsychological function and cerebral glucose metabolism in severe chronic alcoholism. J Clin Exp Neuropsychol. 1997;19:378–85. doi: 10.1080/01688639708403866. [DOI] [PubMed] [Google Scholar]
- Kaplan E. A process approach to neuropsychological assessment. In: Boll T, Bryant BK, editors. Clinical neuropsychology and brain function: research, measurement, and practice. Washington: American Psychological Association; 1988. pp. 129–67. [Google Scholar]
- Karkowski LM, Prescott CA, Kendler KS. Multivariate assessment of factors influencing illicit substance use in twins from female-female pairs. Am J Med Genet. 2000;96:665–70. [PubMed] [Google Scholar]
- Katsugari S, Kunugi H, Sano A, et al. Association between serotonin transporter gene polymorphism and anxiety-related traits. Biol Psychiatry. 1999;45:368–70. doi: 10.1016/s0006-3223(98)00090-0. [DOI] [PubMed] [Google Scholar]
- Keltikangas-Jarvinen L, Elovainio M, Kivimaki M, et al. Association between the type 4 dopamine receptor gene polymorphism and novelty seeking. Psychosom Med. 2003;65:471–6. doi: 10.1097/01.psy.0000041547.31072.25. [DOI] [PubMed] [Google Scholar]
- Keltner D, Shiota MN. New displays and new emotions: a commentary on Rozin and Cohen. Emotion. 2003;3:86–91. doi: 10.1037/1528-3542.3.1.86. [DOI] [PubMed] [Google Scholar]
- Kimbrell TA, George MS, Parekh PI, et al. Regional brain activity during transient self induced anxiety and anger in healthy adults. Biol Psychiatry. 1999;46:454–65. doi: 10.1016/s0006-3223(99)00103-1. [DOI] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach HA, et al. Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci U S A. 2002;99:10825–30. doi: 10.1073/pnas.152112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight RG, Godfrey HP. The role of alcohol-related expectancies in the prediction of drinking behaviour in a stimulated social interaction. Addiction. 1993;88:1111–18. doi: 10.1111/j.1360-0443.1993.tb02130.x. [DOI] [PubMed] [Google Scholar]
- Korbo L. Glial cell in the hippocampus of alcoholics. Alcohol Clin Exp Res. 1999;23:164–8. [PubMed] [Google Scholar]
- Kopelman MD, Wilson BA, Baddeley AD. The autobiographical memory interview: a new assessment of autobiographical and personal semantic memory in amnestic patients. J Clin Exp Neuropsychol. 1989;11:724–44. doi: 10.1080/01688638908400928. [DOI] [PubMed] [Google Scholar]
- Kornreich C, Blairy S, Philippot P, et al. Impaired emotional facial expression recognition in alcoholism compared with obsessive-compulsive disorder and normal controls. Psychiatry Res. 2001a;102:235–48. doi: 10.1016/s0165-1781(01)00261-x. [DOI] [PubMed] [Google Scholar]
- Kornreich C, Blairy S, Philippot P, et al. Deficits in recognition of emotional facial expression are still present in alcoholics after mid-to long-term abstinence. J Stud Alcohol. 2001b;62:533–42. doi: 10.15288/jsa.2001.62.533. [DOI] [PubMed] [Google Scholar]
- Kornreich C, Philippot P, Foisy ML, et al. Impaired emotional facial expression recognition is associated with interpersonal problems in alcoholism. Alcohol Alcohol. 2002;37:394–400. doi: 10.1093/alcalc/37.4.394. [DOI] [PubMed] [Google Scholar]
- Kowner R. Laterality in facial expressions and its effect on attributions of emotion and personality: a reconsideration. Neuropsychologia. 1995;33:539–59. doi: 10.1016/0028-3932(94)00137-e. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Halliday GM, Svoboda MD, et al. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79:983–98. doi: 10.1016/s0306-4522(97)00083-3. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Araujo I, Rolls ET. Face expression as a reinforcer activates the orbitofrontal cortex in an emotion-related reversal task. NeuroImage. 2001;13(Part 2):S433. [Google Scholar]
- Kruesi MJP, Rapoport JL, Hamburger S, et al. Cerebrospinal fluid monoamine metabolites, aggression, and impulsivity in disruptive behavior disorders of children and adolescents. Arch Gen Psychiatry. 1990;47:419–26. doi: 10.1001/archpsyc.1990.01810170019003. [DOI] [PubMed] [Google Scholar]
- Kubota M, Nakazaki S, Hirai S, et al. Alcohol consumption and frontal lobe shrinkage: study of 1432 non-alcoholic subjects. J Neurol Neurosurg Psychiatry. 2001;71:104–6. doi: 10.1136/jnnp.71.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn K, Meyer K, Nöthen M, et al. Allelic variants of dopamine receptor D4 (DRD4) and serotonin receptor 5HT2c (HTR2c) and temperament factors: replication tests. Am J Med Genet. 1999;88:168–72. [PubMed] [Google Scholar]
- Kumakiri C, Kodama K, Shimizu E, et al. Study of the association between the serotonin transporter gene regulatory region polymorphism and personality traits in a Japanese population. Neurosci Lett. 1999;263:205–7. doi: 10.1016/s0304-3940(99)00098-1. [DOI] [PubMed] [Google Scholar]
- Kumar S, Fleming RL, Morrow AL. Ethanol regulation of gamma-aminobutyric acid A receptors: genomic and nongenomic mechanisms. Pharmacol Ther. 2004;101:211–26. doi: 10.1016/j.pharmthera.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Kuruoglu AC, Arikan Z, Vural G, et al. Single photon emission computerized tomography in chronic alcoholism. Antisocial personality disorder may be associated with decreased frontal perfusion. Br J Psychiatry. 1996;169:348–54. doi: 10.1192/bjp.169.3.348. [DOI] [PubMed] [Google Scholar]
- Laakso MP, Vaurio O, Savolainen L, et al. A volumetric MRI study of the hippocampus in type 1 and 2 alcoholism. Behav Brain Res. 2000;109:177–86. doi: 10.1016/s0166-4328(99)00172-2. [DOI] [PubMed] [Google Scholar]
- Lane SD, Cherek DR, Pietras CJ, et al. Alcohol effects on human risk taking. Psychopharmacology (Berl) 2004;172:68–77. doi: 10.1007/s00213-003-1628-2. [DOI] [PubMed] [Google Scholar]
- Lappalainen J, Long JC, Eggert M, et al. Linkage of antisocial alcoholism to the serotonin 5-HT1B receptor gene in 2 populations. Arch Gen Psychiatry. 1998;55:989–94. doi: 10.1001/archpsyc.55.11.989. [DOI] [PubMed] [Google Scholar]
- Lanza-Kaduce L, Bishop DM, Winner L. Risk/benefit calculations, moral evaluations, and alcohol use: exploring the alcohol-crime connection. Crime Delinq. 1997;43:222–39. [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Ann Rev Neurosci. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23(4–5):727–38. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch K, Bengel D, Heils A, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–31. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Levenston GK, Patrick CJ, Bradley MM, et al. The psychopath as observer: emotion and attention in picture processing. J Abnorm Psychol. 2000;109:373–89. [PubMed] [Google Scholar]
- Levine SC, Levy J. Perceptual asymmetry for chimeric faces across the life span. Brain Cog. 1986;5:291–306. doi: 10.1016/0278-2626(86)90033-3. [DOI] [PubMed] [Google Scholar]
- Lezak MD. 3. New York: Oxford Univ Pr; 1995. Neuropsychological assessment. [Google Scholar]
- Lichter DG, Cummings JL, editors. Frontal-subcortical circuits in psychiatric and neurological disorders. New York: Guilford Pr; 2001. p. 448. [Google Scholar]
- Liotti M, Sava D, Rizzolatti G, et al. Differential hemispheric asymmetries in depression and anxiety: a reaction-time study. Biol Psychiatry. 1991;29:887–99. doi: 10.1016/0006-3223(91)90055-q. [DOI] [PubMed] [Google Scholar]
- Loas G, Otmani O, Lecercle C, et al. Relationships between the emotional and cognitive components of alexithymia and dependency in alcoholics. Psychiatry Res. 2000;96:63–74. doi: 10.1016/s0165-1781(00)00189-x. [DOI] [PubMed] [Google Scholar]
- Loehlin J. Genes and environment in personality development. Newbury Park: Sage Publ; 1992. [Google Scholar]
- Long JC, Knowler WC, Hanson RL, et al. Evidence for genetic linkage to alcohol dependence on chromosome 4 and 11 from an autosome-wide scan in an American Indian population. Am J Med Genet. 1998;81:216–21. doi: 10.1002/(sici)1096-8628(19980508)81:3<216::aid-ajmg2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Malhotra A, Virkkunen M, Rooney W, et al. The association between the dopamine D4 receptor (D4DR) 16 amino acid repeat polymorphism and novelty seeking. Mol Psychiatry. 1996;1:388–91. [PubMed] [Google Scholar]
- Malison RT, Price LH, Berman R, et al. Reduced brain serotonin transporter availability in major depression as measured by (123 I)-2c-carboxy-3β-(4-iodophenyl) tropane and single photon emission computed tomography. Biol Psychiatry. 1998;44:1090–8. doi: 10.1016/s0006-3223(98)00272-8. [DOI] [PubMed] [Google Scholar]
- Mammucari A, Caltagirone C, Ekman P, et al. Spontaneous facial expression of emotions in brain-damaged patients. Cortex. 1988;24:521–33. doi: 10.1016/s0010-9452(88)80046-7. [DOI] [PubMed] [Google Scholar]
- Mandal MK, Mohany A, Pandey R, et al. Emotion-specific processing deficit in focal brain-damage patients. Int J Neurosci. 1996;84(1–4):87–95. doi: 10.3109/00207459608987253. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Malone KM, Diehl GJ, et al. Positron emission tomographic imaging of serotonin activation effects on prefrontal cortex in healthy volunteers. J Cereb Blood Flow Metab. 1996;16:418–26. doi: 10.1097/00004647-199605000-00008. [DOI] [PubMed] [Google Scholar]
- Mann K, Agartz I, Harper C, et al. Neuroimaging in alcoholism: ethanol and brain damage. Alcohol Clin Exp Res. 2001;25:104s–109s. doi: 10.1097/00000374-200105051-00019. [DOI] [PubMed] [Google Scholar]
- Martin PR, Gibbs SJ, Nimmerrichter AA. Brain proton magnetic resonance spectroscopy studies in recently abstinent alcoholics. Alcohol Clin Exp Res. 1995;19:1078–82. doi: 10.1111/j.1530-0277.1995.tb00992.x. [DOI] [PubMed] [Google Scholar]
- Mazzanti CM, Lappalainen J, Long JC, et al. Role of the serotonin transporter promoter polymorphism in anxiety-related traits. Arch Gen Psychiatry. 1998;55:936–40. doi: 10.1001/archpsyc.55.10.936. [DOI] [PubMed] [Google Scholar]
- McGue M. The behavioral genetics of alcoholism. Curr Dir Psychol Sci. 1999;8:109–15. [Google Scholar]
- McHugh SB, Deacon RMJ, Rawlins JNP, et al. Amygdala and ventral hippocampus contribute differentially to mechanisms of fear and anxiety. Behav Neurosci. 2004;118:63–78. doi: 10.1037/0735-7044.118.1.63. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Gray JA. Anxiolytic action on the behavioural inhibition system implies multiple types of arousal contribute to anxiety. J Affect Disorder. 2000;61:161–76. doi: 10.1016/s0165-0327(00)00344-x. [DOI] [PubMed] [Google Scholar]
- Meadows ME, Kaplan RF. Dissociation of autonomic and subjective responses to emotional slides in right hemisphere damage patients. Neuropsychologia. 1994;32:847–56. doi: 10.1016/0028-3932(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Melgaard B, Henriksen L, Ahlgren P, et al. Regional cerebral blood flow in chronic alcoholics measured by single photon emission computerized tomography. Acta Neurol Scand. 1990;82:87–93. doi: 10.1111/j.1600-0404.1990.tb01594.x. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Stolar M, Stevens DE, et al. Familial transmission of substance use disorders. Arch Gen Psychiatry. 1998;55:973–9. doi: 10.1001/archpsyc.55.11.973. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Barros HM, Sakoda L, et al. Alcohol and heightened aggression in individual mice. Alcohol Clin Exp Res. 1998;22:1698–705. [PubMed] [Google Scholar]
- Miczek KA, de Almeida RMM, Rowlett JK, et al. Benzodiazepine/GABAA receptor agonists, self-administered alcohol and aggression in mice. Soc Neurosci Abs. 1998;31:225–8. [Google Scholar]
- Miczek KA, Fish EW, De Bold JF. Neurosteroids, GABAA receptors, and escalated aggressive behavior. Horm Behav. 2003;44:242–57. doi: 10.1016/j.yhbeh.2003.04.002. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Fish EW, DeBold JF, et al. Social and neural determinants of aggressive behavior: novel pharmacotherapeutic targets. Psychopharmacology. 2002;163:434–58. doi: 10.1007/s00213-002-1139-6. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Maxson ST, Faccidomo S, et al. Aggressive behavioral phenotypes in mice. Behav Brain Res. 2001;125:167–81. doi: 10.1016/s0166-4328(01)00298-4. [DOI] [PubMed] [Google Scholar]
- Miczek KA, O’Donnell JM. Alcohol and chlordiazepoxide increase suppressed aggression in mice. Psychopharmacol (Berl) 1980;69:39–44. doi: 10.1007/BF00426519. [DOI] [PubMed] [Google Scholar]
- Miller MW. Generation of neurons in the rat dentate gyrus and hippocampus: effects of prenatal and postnatal treatment with ethanol. Alcohol Clin Exp Res. 1995;19:1500–9. doi: 10.1111/j.1530-0277.1995.tb01014.x. [DOI] [PubMed] [Google Scholar]
- Miranda R, Jr, Meyerson LA, Buchanan TW, et al. Altered emotion-modulated startle in young adults with a family history of alcoholism. Alcohol Clin Exp Res. 2002;26:441–8. [PubMed] [Google Scholar]
- Monot S, Cane E, Burtin JM, et al. Alcohol, criminality, and personality. Ann Med Psychol. 1994;152:316–20. [PubMed] [Google Scholar]
- Monot M, Nixon S, Lovallo W, et al. Altered emotional perception in alcoholics: deficits in affective prosody comprehension. Alcohol Clin Exp Res. 2001;25:362–9. [PubMed] [Google Scholar]
- Moselhy HF, Georgiou G, Kahn A. Frontal lobe changes in alcoholism: a review of the literature. Alcohol Alcohol. 2001;36:357–68. doi: 10.1093/alcalc/36.5.357. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:207–33. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kawashima R, Ito K, et al. Activation of the right inferior frontal cortex during assessment of facial emotion. J Neurophysiol. 1999;82:1610–14. doi: 10.1152/jn.1999.82.3.1610. [DOI] [PubMed] [Google Scholar]
- Narumoto J, Yamada H, Iidaka T, et al. Brain regions involved in verbal or non-verbal aspects of facial emotion recognition. Neuroreport. 2000;11:2571–6. doi: 10.1097/00001756-200008030-00044. [DOI] [PubMed] [Google Scholar]
- Narushima K, Kosier JT, Robinson RG. A reappraisal of poststroke depression, intra- and inter-hemispheric lesion location using meta-analysis. J Neuropsychiat Clin Neurosci. 2003;15:422–30. doi: 10.1176/jnp.15.4.422. [DOI] [PubMed] [Google Scholar]
- Nielsen DA, Virkunnen M, Lappalainen J, et al. A tryptophan hydroxylase gene marker for suicidality and alcoholism. Arch Gen Psychiatry. 1998;55:593–602. doi: 10.1001/archpsyc.55.7.593. [DOI] [PubMed] [Google Scholar]
- Nicolas JM, Catafau AM, Estruch R. Regional cerebral blood flow-SPECT in chronic alcoholism: relation to neuropsychological testing. J Nucl Med. 1993;34:1452–9. [PubMed] [Google Scholar]
- Noble E, Ozkaragoz T, Ritchie T, et al. D2 and D4 dopamine receptor polymorphisms and personality. Am J Med Genet. 1998;81:257–67. [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Foroud T, Flury L, et al. Is there a genetic relationship between alcoholism and depression? Alcohol Res Health. 2002;26:233–40. [PMC free article] [PubMed] [Google Scholar]
- Ohman A, Soares JJ. Emotional conditioning to masked stimuli: expectancies for aversive outcomes following nonrecognized fear-relevant stimuli. J Exp Psychol Gen. 1998;127:69–82. doi: 10.1037//0096-3445.127.1.69. [DOI] [PubMed] [Google Scholar]
- Ono Y, Manki H, Yoshimura K, et al. Association between dopamine D4 receptor (D4DR) exon III polymorphism and novelty seeking in Japanese subjects. Am J Med Genet. 1997;74:501–3. [PubMed] [Google Scholar]
- Oscar-Berman M. Neuropsychological vulnerabilities in chronic alcoholism. In: Noronha A, Eckardt MJ, Warren K, editors. Review of NIAAA’s neuroscience and behavioral research portfolio. Volume monograph nr 34. Bethesda: US Department of Health and Human Services; 2000. pp. 437–71. [Google Scholar]
- Oscar-Berman M, Hancock M, Mildworf B, et al. Emotional perception and memory in alcoholism and aging. Alcohol Clin Exp Res. 1990;14:383–93. doi: 10.1111/j.1530-0277.1990.tb00491.x. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, McNamara P, Freedman M. Delayed-response tasks: parallels between experimental ablation studies and findings in patients with frontal lesions. In: Levin HS, Eisenberg HM, Benton AL, editors. Frontal lobe function and injury. New York: Oxford Univ Pr; 1991. pp. 231–55. [Google Scholar]
- Oscar-Berman M, Schendan HE. Asymmetries of brain function in alcoholism: relationship to aging. In: Obler L, Connor LT, editors. Neurobehavior of language and cognition: studies of normal aging and brain damage. New York: Kluwer Academic Publ; 2000. pp. 213–40. [Google Scholar]
- Oscar-Berman M, Shagrin B, Evert DL. Impairments of brain and behavior: the neurological effects of alcohol. Alcohol Health Res World. 1997;21:65–75. [PMC free article] [PubMed] [Google Scholar]
- Panksepp J. Affective neuroscience. New York: Oxford Univ Pr; 1998. [Google Scholar]
- Paradiso S, Chemerinski E, Yazici KM, et al. Frontal lobe syndrome reassessed: comparison of patients with lateral or medial frontal brain damage. J Neurol Neurosurg Psychiatry. 1999;67:664–7. doi: 10.1136/jnnp.67.5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegna GD, Caldara-Schnetzer AS, Perrg SH, et al. Is the right amygdala involved in visuospatial memory? Evidence from MRI volumetric measures. Eur Neurol. 2002;47:148–55. doi: 10.1159/000047973. [DOI] [PubMed] [Google Scholar]
- Pentney R, Mullan B, Felong A, et al. The total numbers of cerebellar granule neurons in young and aged Fischer 344 and Wistarkyoto rats do not change as a result of lengthy ethanol treatment. Cerebellum. 2002;1:79–89. doi: 10.1080/147342202753203113. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Desmond JE, et al. Thinning of the corpus callosum in older alcoholic men: a magnetic resonance imaging study. Alcohol Clin Exp Res. 1996;20:752–7. doi: 10.1111/j.1530-0277.1996.tb01682.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, et al. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcohol Clin Exp Res. 1992;16:1078–89. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Serventi KL, et al. Corpus callosum, pons and cortical white matter in alcoholic women. Alcohol Clin Exp Res. 2002;26:400–6. [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan E. Microstructural but not macrostructural disruption of white matter in women with chronic alcoholism. NeuroImage. 2002;15:708–18. doi: 10.1006/nimg.2001.1018. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, et al. In vivo detection and functional correlates of white matter microstructural disruption in chronic alcoholism. Alcohol Clin Exp Res. 2000;24:1214–21. [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, et al. Longitudinal changes in magnetic resonance imaging brain volume in abstinent and relapsed alcoholics. Alcohol Clin Exp Res. 1995;19:1177–91. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, et al. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res. 1997;21:521–9. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Philippot P, Kornreich C, Blairy S, et al. Alcoholics’ deficits in the decoding of emotional facial expression. Alcohol Clin Exp Res. 1999;23:1031–8. [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, et al. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–28. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Phillips SC, Harper CG, Kril J. A quantitative histological study of the cerebellar vermis in alcoholic patients. Brain. 1987;110:301–14. doi: 10.1093/brain/110.2.301. [DOI] [PubMed] [Google Scholar]
- Pihl RO, Peterson JB. A biobehavioural model for the inherited predisposition to alcoholism. Alcohol Alcohol Suppl. 1991;1:151–6. [PubMed] [Google Scholar]
- Pitkänen A. Connectivity of the rat amygdaloid complex. In: Aggleton JP, editor. The amygdala: a functional analysis. 2. Oxford: Oxford Univ Pr; 2000. pp. 31–115. [Google Scholar]
- Pitkänen A, Pikkarainen M, Nurminen N, et al. Reciprocal connections between the amygdala and the hippocampus formation, perirhinal cortex, and postrhinal cortex in rat. Ann N Y Acad Sci. 2000;911:369–91. doi: 10.1111/j.1749-6632.2000.tb06738.x. A review. [DOI] [PubMed] [Google Scholar]
- Pogue-Geile M, Ferrell R, Deka R, et al. Human novelty-seeking personality traits and dopamine D4 receptor polymorphisms: a twin and genetic association study. Am J Med Genet. 1998;81:44–8. [PubMed] [Google Scholar]
- Raine A, Meloy JR, Bihrle S, et al. Reduced prefrontal and increased subcortical brain functioning assessed using positron emission tomography in predatory and affective murderers. Behav Sci Law. 1998;16:319–32. doi: 10.1002/(sici)1099-0798(199822)16:3<319::aid-bsl311>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Ratti MT, Bo P, Giardini A, et al. Chronic alcoholism and the frontal lobe which executive functions are impaired? Acta Neurol Scand. 2002;105:276–81. doi: 10.1034/j.1600-0404.2002.0o315.x. [DOI] [PubMed] [Google Scholar]
- Ratti MT, Soragna D, Sibilla L, et al. Cognitive impairment and cerebral atrophy in “heavy drinkers”. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:243–58. doi: 10.1016/s0278-5846(98)00103-1. [DOI] [PubMed] [Google Scholar]
- Ravaglia S, Costa A, Ratti MT. Cognitive impairment and central motor conduction time in chronic alcoholics. Funct Neurol. 2002;17:83–6. [PubMed] [Google Scholar]
- Reich T, Edenberg HJ, Goate A, et al. Genome-wide search for genes affecting the risk for alcohol dependence. Am J Med Genet. 1998;81:207–15. [PubMed] [Google Scholar]
- Richardson MP, Strange BA, Dolan RJ. Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nature Neurosci. 2004;47:278–85. doi: 10.1038/nn1190. [DOI] [PubMed] [Google Scholar]
- Ricketts M, Hamer R, Sage J, et al. Association of a serotonin transporter gene promoter polymorphism with harm avoidance behavior in an elderly population. Psychiatr Genet. 1998;8:41–4. doi: 10.1097/00041444-199800820-00001. [DOI] [PubMed] [Google Scholar]
- Roberto M, Nelson TE, Ur CL, et al. Long-term potentiation in the rat hippocampus is reversibly depressed by chronic intermittent ethanol exposure. J Neurophysiol. 2002;87:2385–97. doi: 10.1152/jn.2002.87.5.2385. [DOI] [PubMed] [Google Scholar]
- Robinson RG, Starkstein SE. Mood disorders following stroke: new finding and future direction. J Geriatr Psychiatry. 1989;22:1–15. [PubMed] [Google Scholar]
- Rolls ET. Precis of the brain and emotion. Behav Brain Sci. 2000;23:177–91. doi: 10.1017/s0140525x00002429. [DOI] [PubMed] [Google Scholar]
- Rosenthal NE, Mazzanti CM, Barnett RL, et al. Role of serotonin transporter repeat length polymorphism (5-HTTLPR) in seasonality and seasonal affective disorder. Mol Psychiatry. 1998;3:175–7. doi: 10.1038/sj.mp.4000360. [DOI] [PubMed] [Google Scholar]
- Ross ED. Right hemisphere’s role in language, affective behavior and emotion. Trends Neurosci. 1984;7:342–6. [Google Scholar]
- Ross ED, Mesulam MM. Dominant language functions of the right hemisphere? Prosody and emotional gesturing. Arch Neurol. 1979;36:144–8. doi: 10.1001/archneur.1979.00500390062006. [DOI] [PubMed] [Google Scholar]
- Sackeim HA, Gur RC, Saucy MC. Emotions are expressed more intensely on the left side on the face. Science. 1978;202:433–5. doi: 10.1126/science.705335. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ESL, Lopez De Armentia M, et al. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–34. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Sander T, Harms H, Dufeu P, et al. Dopamine D4 receptor exon III alleles and variation of novelty seeking in alcoholics. Am J Med Genet. 1997;74:483–7. doi: 10.1002/(sici)1096-8628(19970919)74:5<483::aid-ajmg5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Santin LJ, Rubio S, Begega A, et al. Effects of chronic alcohol consumption on spatial reference and working memory tasks. Alcohol. 2000;20:149–59. doi: 10.1016/s0741-8329(99)00070-1. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Changes in functional connectivity in orbitofrontal cortex and basolateral amygdala during learning and reversal training. J Neurosci. 2000;20:5179–89. doi: 10.1523/JNEUROSCI.20-13-05179.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD. The cerebrocerebellar system. Int Rev Neurobiol. 1997;41:31–60. doi: 10.1016/s0074-7742(08)60346-3. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. The role of the cerebellum in affect and psychosis. J Neurolinguistics. 2000;13:189–214. [Google Scholar]
- Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar-cognitive affective syndromes. J Neuropsychiat Clin Neurosci. 2004;16:367–78. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman J. The cerebellar cognitive affective syndrome. Brain. 1998;121:561–79. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Schneider F, Habel U, Wagner M, et al. Subcortical correlates of craving in recently abstinent alcoholic patients. Am J Psychiatry. 2001;158:1075–83. doi: 10.1176/appi.ajp.158.7.1075. [DOI] [PubMed] [Google Scholar]
- Schulte T, Pfefferbaum A, Sullivan EV. Parallel interhemispheric processing in aging and alcoholism: relation to corpus callosum size. Neuropsychologia. 2004;42:257–71. doi: 10.1016/s0028-3932(03)00155-6. [DOI] [PubMed] [Google Scholar]
- Scicli AP, Petrovich GD, Swanson LW, et al. Contextual fear conditioning is associated with lateralized expression of the immediate early gene c-fos in the central and basolateral amygdalar nuclei. Behav Neurosci. 2004;118:5–14. doi: 10.1037/0735-7044.118.1.5. [DOI] [PubMed] [Google Scholar]
- Seitz D, Widmann U, Seeger U. Localized protein magnetic resonance spectroscopy of the cerebellum in detoxifying alcoholics. Alcohol Clin Exp Res. 1999;23:158–63. [PubMed] [Google Scholar]
- Semmes J. Hemispheric specialization: a possible clue to mechanism. Neuropsychologia. 1968;6:11–26. [Google Scholar]
- Seto MC, Barbaree HE. The role of alcohol in sexual aggression. Clin Psychol Rev. 1995;15:545–66. [Google Scholar]
- Shear PK, Jernigan TL, Butters N. Volumetric magnetic resonance imaging quantification of longitudinal brain changes in abstinent alcoholics. Alcohol Clin Exp Res. 1994;18:172–6. doi: 10.1111/j.1530-0277.1994.tb00899.x. [DOI] [PubMed] [Google Scholar]
- Spence S, Shapiro D, Zaidel E. The role of the right hemisphere in the physiological and cognitive components of emotional processing. Psychophysiology. 1996;33:112–22. doi: 10.1111/j.1469-8986.1996.tb02115.x. [DOI] [PubMed] [Google Scholar]
- Sperling W, Frank H, Martus P, et al. The concept of abnormal hemispheric organization in addiction research. Alcohol Alcohol. 2000;35:394–9. doi: 10.1093/alcalc/35.4.394. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Maguire EA, Burgess N. Hippocampal amnesia. Neurocase. 2001;7:357–82. doi: 10.1076/neur.7.5.357.16245. [DOI] [PubMed] [Google Scholar]
- Springer SP, Deutch G. Left brain right brain: perspectives from cognitive neuroscience. New York: Freeman; 1998. [Google Scholar]
- Starkstein SE, Pearlson GD, Boston J, et al. Mania after brain injury: a controlled study of causative factors. Arch Neurol. 1987;44:1069–73. doi: 10.1001/archneur.1987.00520220065019. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Robinson RG, Honig MA, et al. Mood changes after right-hemisphere lesions. Br J Psychiatry. 1989;155:79–85. doi: 10.1192/bjp.155.1.79. [DOI] [PubMed] [Google Scholar]
- Strobel A, Wehr A, Michel A, et al. Association between the D4 receptor (DRD4) exon III polymorphism and measures of novelty seeking in a German population. Mol Psychiatry. 1999;4:378–84. doi: 10.1038/sj.mp.4000535. [DOI] [PubMed] [Google Scholar]
- Sullivan EV. Human brain vulnerability to alcoholism: evidence from neuroimaging studies. In: Noronha A, Eckardt M, Warren K, editors. Review of NIAAA’s Neuroscience and Behavioral Research Portfolio (NIAAA Research Monograph nr 34) Bethesda: National Institutes of Health; 2000. pp. 473–508. [Google Scholar]
- Sullivan EV. Compromised pontocerebellar and cerebello-thalamocortical systems: speculations on their contributions to cognitive and motor impairment in nonamnesic. Alcohol Clin Exp Res. 2003;27:1409–19. doi: 10.1097/01.ALC.0000085586.91726.46. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Fifield WJ, Kennedy MA, et al. No association between novelty seeking and the type 4 dopamine receptor gene (DRD4) in two New Zealand samples. Am J Psychiatry. 1998;155:98–101. doi: 10.1176/ajp.155.1.98. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Harding AJ, Pentney R, et al. Disruption of frontocerebellar circuitry and function in alcoholism. Alcohol Clin Exp Res. 2003;27:301–9. doi: 10.1097/01.ALC.0000052584.05305.98. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcohol Clin Exp Res. 2000;24:611–21. [PubMed] [Google Scholar]
- Tarter RE, Hegedus AM, Van Thiel DH, et al. Portal-systemic encephalopathy: neuropsychiatric manifestations. Int J Psychiat Med. 1985;15:265–75. doi: 10.2190/lyef-yvy4-hdrl-8uew. [DOI] [PubMed] [Google Scholar]
- Taylor SP, Chermack ST. Alcohol, drugs, and human physical aggression. J Stud Alcohol. 1993;11:78–88. doi: 10.15288/jsas.1993.s11.78. [DOI] [PubMed] [Google Scholar]
- Theodore J, Waterman B, Health A, et al. Genetic and environmental effects on offspring alcoholism: new insights using an offspring-of-twins design. Arch Gen Psychiatry. 2003;60:1265–72. doi: 10.1001/archpsyc.60.12.1265. [DOI] [PubMed] [Google Scholar]
- Townshend JM, Duka T. Mixed emotions: alcoholics’ impairments in the recognition of specific emotional facial expressions. Neuropsychologia. 2003;41:773–82. doi: 10.1016/s0028-3932(02)00284-1. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Meyer JM, et al. Co-occurrence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities. Arch Gen Psychiatry. 1998;55:967–72. doi: 10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- Tucker DM, Williamson PA. Asymmetric neural control systems in human self-regulation. Psychol Rev. 1984;91:185–215. [PubMed] [Google Scholar]
- Uhl GR, Grow RW. The burden of complex genetics in brain disorders. Arch Gen Psychiatry. 2004;61:223–9. doi: 10.1001/archpsyc.61.3.223. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Liu QR, Walther D, et al. Polysubstance abuse-vulnerability genes: genome scans for association, using 1004 subjects and 1494 single-nucleotide polymorphism. Am J Hum Genet. 2001;69:1290–300. doi: 10.1086/324467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff syndrome and related neurologic disorders due to alcoholism and malnutrition. 2. Philadelphia: FA Davis Co; 1989. [Google Scholar]
- Virkunnen M, Kallio E, Rawlings R, et al. Personality profiles and state aggressiveness in Finnish alcoholic, violent offenders, fire setters, and healthy volunteers. Arch Gen Psychiatry. 1994;51:28–33. doi: 10.1001/archpsyc.1994.03950010028004. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wang GJ, et al. Decreased brain metabolism in neurologically intact healthy alcoholics. Am J Psychiatry. 1992;149:1016–22. doi: 10.1176/ajp.149.8.1016. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hilzemann R, Wolf AP, et al. Acute effects of ethanol on regional brain glucose metabolism and transport. Psychiatr Res. 1990;35:39–48. doi: 10.1016/0925-4927(90)90007-s. [DOI] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, et al. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of finding from neuroimaging. NeuroImage. 2003;19:513–31. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Walker DW, Freund G. Impairment of shuttle box avoidance learning following prolonged alcohol consumption in rats. Physiol Behav. 1971;7:773–8. doi: 10.1016/0031-9384(71)90147-8. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Roque CT. Functional importance of ventricular enlargement and cortical atrophy in healthy subjects and alcoholics as assessed with PET, MR imaging, and neuropsychologic testing. Radiology. 1993;186:59–65. doi: 10.1148/radiology.186.1.8416587. [DOI] [PubMed] [Google Scholar]
- Weddell RA. Effects of subcortical lesion site on human emotional behavior. Brain Cogn. 1994;25:161–93. doi: 10.1006/brcg.1994.1029. [DOI] [PubMed] [Google Scholar]
- West JR, Pierce DR. Perinatal alcohol exposure and neuronal damage. In: West JR, editor. Alcohol and brain development. New York: Oxford Univ Pr; 1986. pp. 120–57. [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, et al. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18:411–18. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, Matthews DB, Best PJ. Ethanol, memory, and hippocampal function: a review of recent findings. Hippocampus. 2000;10:88–93. doi: 10.1002/(SICI)1098-1063(2000)10:1<88::AID-HIPO10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Wildgruber D, Pihan H, Ackermann H, et al. Dynamic brain activation during processing of emotional intonation: influence of acoustic parameters, emotional valence, and sex. Neuroimage. 2002;15:856–69. doi: 10.1006/nimg.2001.0998. [DOI] [PubMed] [Google Scholar]
- Wittling W, Roschmann R. Emotion-related hemispheric asymmetry: subjective emotional response to laterally presented films. Cortex. 1993;29:431–48. doi: 10.1016/s0010-9452(13)80252-3. [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Joh K, Ohta T, et al. Neurons but not glial cells show reciprocal imprinting of sense and antisense transcripts of Ube3aI. Hum Mol Genet. 2003;12:837–47. doi: 10.1093/hmg/ddg106. [DOI] [PubMed] [Google Scholar]


