Abstract
Increased attention has been given to the alternatives to stimulants in the treatment of attention deficit hyperactivity disorder (ADHD) in both adults and children. This short-term, double-blind trial was designed to evaluate the extended-release form of bupropion in adult subjects meeting DSM-IV and the Utah Diagnostic Criteria for ADHD. Outcome measures were the Clinical Global Impressions-Improvement (CGI-I) and the Wender-Reimherr Adult Attention Deficit Disorder Scale (WRAADDS), which assesses adult ADHD symptoms. Outcome (defined by the CGI-I, average WRAADDS scores, or a 50% improvement on the WRAADDS) favored bupropion SR over placebo, but achieved statistical significance on only one, post hoc measure. Other measures showed trends for improvement with bupropion. Given the small size of this study, these findings deserve further exploration.
Keywords: bupropion SR, ADHD, adult, controlled study
Introduction
Attention deficit hyperactivity disorder (ADHD) is the most common psychiatric disorder in childhood, and recent research has documented its persistence into adulthood (Faraone et al 2000). The stimulant medications methylphenidate and dextroamphetamine are the most widely accepted and successfully tested treatments for ADHD in children and adults (Wender et al 1985; Wender 1995; Cyr and Brown 1998; Wender 1998; Wilens and Spencer 2000). However, a number of concerns including questions of abuse, problems in administration, lack of efficacy in some patients, and controversies regarding the stimulants have limited the use of these medications in adults with suspected ADHD. These factors may contribute to an undertreatment of ADHD in adults. Consequently, there have been efforts to find alternative nonstimulant treatment approaches for ADHD.
One of the most commonly proposed alternatives has been the antidepressants, particularly those with noradrenergic activity. There have been multiple reports on the use of such antidepressants for the treatment of ADHD in both pediatric and adult populations. These include imipramine (Huessy and Wright 1970; Rapoport et al 1974), desipramine (Donnelly et al 1986; Biederman et al 1989), nortriptyline (Spencer et al 1993), venlafaxine (Hedges et al 1995), protriptyline (Wilens et al 1996), and atomoxetine (Spencer et al 1998; Michelson et al 2003).
There have been a number of such reports on bupropion (Wender and Reimherr 1990; Conners et al 1996; Wilens et al 2001). Although the mechanism of action of bupropion is unknown, it seems to potentiate central nervous system activity of both norepinephrine and dopamine. It is a weak inhibitor of both dopamine and norepinephrine reuptake (Richelson 1991), but it may potentiate the activity of these two neurotransmitters through other, more complex, mechanisms (Ascher et al 1995). Among these antidepressants, only bupropion appears to have this dual dopamine–norepinephrine mechanism of action (Golden et al 1988; Richelson 1991). In this regard, the pharmacologic activity of bupropion is more similar to the presumed action of stimulants (Patrick et al 1987; Heal et al 1989). A number of theories regarding the etiology of ADHD have suggested that this dual mechanism of action is important in its treatment (Zametkin and Rapoport 1987; Hechtman 1994).
Despite widespread use of bupropion in the treatment of ADHD, there are only limited data from controlled studies regarding its effectiveness. Documenting its efficacy in ADHD is not only of clinical interest, but might lead to new avenues of research regarding the etiology of ADHD. In the past, we have reported on an initial exploratory, open-label study on the use of bupropion in adult ADHD (Wender and Reimherr 1990). In this study of 19 adults previously treated with either stimulants or monoamine oxidase inhibitors, 14 experienced moderate to marked benefit from bupropion. Ten of these patients chose to remain on bupropion rather than their former medication. These results were in part replicated by a controlled study by Wilens et al (2001).
The first of these two studies employed the immediate-release form of bupropion that usually requires three-times-daily administration. The sustained-release (SR) formulation of bupropion (used in the second study) is bioequivalent to the immediate-release formulation. Following multiple-day administration, the sustained-release formulation of bupropion produces peak plasma concentrations of bupropion that are approximately 85% of those achieved with the immediate-release formulation. In theory, this should provide fewer peak-related side effects and possibly a lower risk of seizures (PDR 2001). This alternative form of bupropion may make the medication more acceptable in the treatment of ADHD. The sustained-release formulation is more convenient to use, and Reimherr et al (1998) found antidepressant efficacy in once-daily dosing.
On the basis of these factors, we conducted a controlled study of bupropion SR to evaluate its short-term efficacy in adults with ADHD.
Methods
This study consisted of a 1-week baseline phase with single-blind placebo administration followed by a 6-week randomized, double-blind, placebo-controlled, parallel-design phase. The study was reviewed and approved by the Institutional Review Board of the University of Utah Health Sciences Center.
In the double-blind portion, 60% of the patients were assigned to bupropion SR and 40% to placebo. (This uneven assignment was done to maximize the number of initial bupropion SR responders enrolled in a long-term, follow-up study.) Subjects were recruited from clinic patients, referrals, and a limited amount of public solicitations. All subjects received a comprehensive description of the study and they provided informed consent before enrollment. Outpatient subjects aged at least 18 years were required to meet not only DSM-IV but also the more restrictive Utah Criteria for ADHD in adults (Wender 1995) and to have a minimum score of 15 on the Wender-Reimherr Adult Attention Deficit Disorder Scale (WRAADDS).
The Utah Criteria were developed to identify a homogeneous “core” ADHD population and are quite restrictive. They involve assessing both childhood and adult signs and symptoms, preferably using the subjects’ parents to assess their childhood behavior and a “significant other” to assess current symptoms. The childhood criteria are a childhood history consistent with ADHD in childhood as defined by A or B:
Narrow criteria. The individual met DSM-IV criteria for ADHD in childhood (6 of the 9 signs or symptoms of inattention and/or 6 of the 9 signs or symptoms of hyperactivity/impulsivity).
Broad criteria. The individual had a history of attention deficits and hyperactivity, and at least one of the following: behavior problems in school, impulsivity, over-excitability, or temper outbursts. The patient also had a “Parent Rating Scale” or “Wender Utah Rating Scale” score in the 95th percentile.
The adult criteria are at least moderate impairment of both motor hyperactivity and attentional difficulties, plus at least two of the following characteristics: affective lability, inability to complete tasks/disorganization, hot temper, emotional overreactivity/stress intolerance, and impulsivity. The intensity of these symptoms was assessed using the WRAADDS.
Any history of stimulant drug abuse or other recent substance abuse would exclude a patient from clinical trials involving stimulants. The Utah Criteria also exclude patients with the following characteristics or disorders:
bipolar and depressive mood disorders
signs and symptoms of schizophrenic spectrum disorders
borderline personality disorder
antisocial personality disorder.
Subjects were required to have a spouse or close family member who was willing to attend visits with the patients to report on their symptoms. Information supplied by family members supplemented the patient’s report and was incorporated as appropriate in scoring both the WRAADDS and Clinical Global Impressions-Improvements scale (CGI-I). This was done in a joint interview. (It is our experience that the use of a family member significantly improves the validity and reliability of the assessment of adult patients with ADHD.) Additionally, subjects had to have at least moderate impairment in one area of social adjustment as measured by the Weissman Social Adjustment Scale (WSAS) (Weissman 1975). In addition to the exclusion factors in the Utah Criteria, eating disorders, seizure disorders, history of significant head injury, and situational stresses that were severe enough to confuse interpretation of outcome measures were exclusionary factors. Women who were pregnant or breast feeding, subjects under custody of the criminal justice system, subjects with a history of treatment with bupropion, and subjects at risk for suicide were excluded. Finally, individuals with other axis I disorders were excluded.
To avoid confounding the antidepressant effects of bupropion with its putative properties in ADHD, we were particularly concerned about excluding patients with significant depressive symptoms. Consequently, patients scoring over 15 on the Hamilton Depression Scale (HAM-D), having a score of 8 or more on the sum of HAM-D items #1, #2, #3, and #7, or meeting DSM-IV criteria for current major depression or dysthymia were excluded. However, patients with a history of a single episode of major depression associated with a significant life stress were allowed in the study.
The WRAADDS was used to assess symptoms specific to ADHD. This is a 28-point, clinician-administered, semi-structured interview containing seven items: attention difficulties, hyperactivity/restlessness, temper, mood instability, emotional overreactivity, disorganization, and impulsivity. The WRAADDS (Reimherr et al 2003) is a modification of the Targeted Attention Deficit Disorder Scale (Wender 1995). It is currently available from the authors and will be published in the near future. The physician-rated CGI-I scale was used to assess general improvement. To assess social functioning, the WSAS was administered by a rater experienced in its use. The WSAS is a clinician-administered scale addressing overall social adjustment as well as five specific components: work, social leisure, extended family, marriage, and parental functioning (Weissman 1975).
Bupropion SR was initiated at 100 mg/day and titrated by 100-mg/day increments based on clinical response and as tolerated to a maximum dose of 400 mg/day (200 mg twice daily), usually reached after 21 days on study medication. An attempt was made to increase the dose of bupropion over the first 3 weeks to the maximum tolerated dose or until a significant response was produced as assessed by the CGI-I scale. Dosing was flexible and could be either once or twice daily, depending upon response, adverse effects, and patient preference. However, if the total daily dose exceeded 200 mg, it was divided between two daily doses. The average daily dose was 298 mg/day at the end of the study.
Clinic visits were done at days 0, 7, 14, 21, 35, and 49. After the conclusion of the study, patients were allowed to enter a 6-month open-label study. At the conclusion of either portion of the overall study, patients were followed until stable and then treated as private clinic patients or referred to an outside clinic.
Patients who had a CGI-I score of 1 or 2 (very much or much improved) or who had a reduction in the WRAADDS of 50% or more were categorized as treatment responders. Fisher’s exact test was used to compare the proportion of responders on placebo versus bupropion SR. To compare study groups on continuous variables a repeated measures ANOVA (using the screening visit and the last double-blind visit) was performed. For comparison of groups on continuous variables at specific time intervals, the Mann-Whitney (Wilcoxon) test was used. All statistical tests were two-tailed with statistical significance at the p = 0.05 level. All patients with at least one outcome measure during the double-blind period were included using a last-visit-carried-forward design. Statistical tests were performed using the SPSS 11.5 statistical package.
Results
Fifty-nine patients (43 male, 16 female) signed informed consent for entry into the study. All but two patients were assessed as having substantial symptoms in both attentional and hyperactive areas. These two patients had primarily attentional symptoms. The patients assigned to two treatment groups (placebo or bupropion SR) did not differ in demographic or pretreatment measures (Table 1). There were mild elevations in anxiety and depression as indicated by average HAM-D scores of 10.1 ± 4.9 (all scores were below 15) and average Hamilton Anxiety Scale (HAM-A) scores of 11.9 ± 5.0. Fourteen patients (24%) scored above 15 on the HAM-A. Seventy-nine percent of patients were experiencing at least moderate problems in their overall social adjustment, a non-surprising number given that one requirement for admission was at least moderate impairment in one area of social adjustment. Work and marriage were the most common problem areas.
Table 1.
Pretreatment demographic and clinical characteristics of all adults enrolled in the study
| Variable | Bupropion SR | Placebo | Total |
|---|---|---|---|
| N | 35 | 24 | 59 |
| Male/female | 25/10 | 18/6 | 43/16 |
| Age (y) (mean ± SD) | 34.3 ± 14.8 | 34.6 ± 11.2 | 34.4 ± 13.4 |
| GAF (mean ± SD) | 53.3 ± 4.6 | 54.6 ± 3.1 | 53.9 ± 4.1 |
| HAM-D (mean ± SD) | 10.0 ± 4.7 | 10.2 ± 5.2 | 10.1 ± 4.9 |
| HAM-A (mean ± SD) | 12.1 ± 5.2 | 11.5 ± 4.7 | 11.9 ± 5.0 |
| Parent Rating Scale (mean ± SD) | 19.8 ± 5.5 | 19.5 ± 5.0 | 19.6 ± 5.1 |
| Screening WRAADDS (total) (mean ± SD) | 20.3 ± 3.7 | 20.2 ± 3.7 | 20.2 ± 3.7 |
| WRAADDS subscales: percentage of patients with at least moderate impairment | |||
| Attention difficulties | 100 | 100 | 100 |
| Hyperactivity | 91 | 96 | 93 |
| Temper | 77 | 79 | 78 |
| Mood instability | 89 | 96 | 92 |
| Overreactivity | 91 | 88 | 90 |
| Disorganization | 97 | 100 | 98 |
| Impulsivity | 94 | 96 | 95 |
| Weissman Social Adjustment Scale: percentage of patients with at least moderate impairment | |||
| Work | 67 | 60 | 64 |
| Social leisure | 50 | 45 | 48 |
| Extended family | 39 | 20 | 30 |
| Marital | 75 | 47 | 62 |
| Parental | 20 | 14 | 17 |
| Overall functioning | 83 | 74 | 79 |
Abbreviations: GAF, Global Assessment of Functioning Scale; HAM-A, Hamilton Anxiety Scale; HAM-D, Hamilton Depression Scale-17 item; WRAADDS, Wender-Reimherr Adult Attention Deficit Disorder Scale.
Of the 59 patients who entered the study, 47 provided outcome data during the double-blind period. Patients dropping out either did not complete the single-blind evaluation or did not furnish outcome data during the double-blind period following randomization. There were no significant pretreatment differences between these patients and those continuing in the study. Furthermore, there was no evidence of adverse effects leading to study withdrawal.
Data were collected on vital signs and side effects at each visit. There were no significant adverse effects and the medication was well tolerated.
Two criteria were used to define response: a 50% or more reduction in the total WRAADDS score, and ratings of “much” to “very much” improved on the CGI-I. Table 2 shows the outcome measures for the 47 patients who completed the double-blind period. Compared with those receiving placebo, patients on bupropion SR were more likely to show a 50% improvement in their WRAADDS scores from the screening visit to the last visit in the double-blind period (39% versus 11%; p < 0.05, Fisher’s exact test). Conversely, if improvement was measured from the end of the single-blind period to last visit, the difference was no longer significant (32% versus 11%; p = 0.15, Fisher’s exact test). On the CGI-I, more bupropion SR than placebo recipients were improved (41% versus 22%; p = 0.15, Fisher’s exact test), but this difference was not statistically significant.
Table 2.
Outcome values at the end of double-blind treatment
| Variable | Bupropion SR | Placebo | p-value |
|---|---|---|---|
| N | 29 | 18 | |
| GAF (mean ± SD) | 57.5 ± 8.1 | 56.2 ± 3.6 | NS |
| CGI-I (% much or very much improved) | 41 | 22 | = 0.15 |
| HAM-A (mean ± SD) | 10.2 ± 5.4 | 8.1 ± 4.1 | NS |
| WRAADDS (mean ± SD) (total) | 12.9 ± 5.6 | 14.7 ± 5.1 | NS |
| 50% improvement (%) | 39 | 11 | < 0.05 |
| WRAADDS subscales: percentage improvement over screening evaluation | |||
| Attention difficulty | 26 | 17 | NS |
| Hyperactivity | 28 | 12 | NS |
| Temper | 44 | 46 | NS |
| Mood instability | 41 | 41 | NS |
| Overreactivity | 45 | 34 | NS |
| Disorganization | 29 | 16 | = 0.06 |
| Impulsivity | 33 | 27 | NS |
Abbreviations: CGI-I, Clinical Global Impressions-Improvement; GAF, Global Assessment of Functioning; HAM-A, Hamilton Anxiety Scale; NS, not significant; WRAADDS, Wender-Reimherr Adult Attention Deficit Disorder Scale.
There was a decrease in average scores for all seven items of the WRAADDS. However, both patients on placebo and those on bupropion SR demonstrated this improvement, and the difference between the two groups was not significant on this measure.
Figure 1 shows the total WRAADDS for the double-blind assessment period for the 18 placebo and 29 bupropion SR patients. This graph does not carry forward data on patients who dropped out. Both groups showed lower WRAADDS scores through the first week of the double-blind period. Thereafter, the placebo patients showed no more change, while the bupropion SR patients improved an additional 3.5 points. The subjects in the active treatment arm averaged 20.3 at the screening evaluation and this improved to 12.9 for an average 36% improvement. The placebo subjects averaged 19.7 at the screening evaluation and showed a 25% improvement to 14.7. Repeated measures ANOVA (using only screen and last visit) uncovered a main effect for time (p < 0.0005) but no drug-by-time interaction (p < 0.38). The difference between the placebo and bupropion SR groups on the total WRAADDS scores at the end of the double-blind period did not achieve statistical significance.
Figure 1.
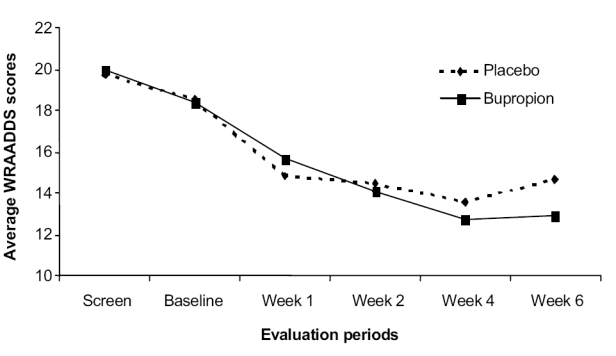
Change in average WRAADDS (Wender-Reimherr Adult Attention Deficit Disorder Scale) scores.
Discussion
Although bupropion SR produced more improvement than placebo in reducing ADHD symptoms in this short-term, double-blind study, statistical analysis indicated that these were nonsignificant numerical trends favoring bupropion SR. There was, however, a significant post hoc difference when comparing the percentage of responders defined by a 50% WRAADDS improvement over the screening evaluation (Table 2), although this represents an unconventional manner of analysis. No statistically significant differences were found between bupropion and placebo a priori. This limited statistical significance stands in contrast to the more positive studies of bupropion found in the literature.
Most studies that employ a single-blind placebo phase use the results at the end of the single-blind period to measure change over the course of the study. This is done partly to give researchers an extended opportunity to remove inappropriate patients. Additionally, it is generally believed that the single-blind phase may increase the power of the study. We had initially examined this data set employing the scores at the end of the single-blind phase to measure change over the course of the study. However, after producing a graph of the WRAADDS for the study visits, and noting the changes during the single-blind week on placebo, we decided to measure improvement from both the beginning and the end of the single-blind placebo period. In contrast to expectations, the single-blind data actually reduced the differences found in the study.
The difference between medication and placebo conditions was significant when comparing a 50% WRAADDS improvement over the screening period but was not significant when using a CGI-I of “much” or “very much” improved to define responders. Treatment differences as measured by the average score on the WRAADDS and the individual WRAADDS items also failed to achieve significance.
This limited statistical significance contrasts with previously reported studies evaluating bupropion in ADHD. Five factors may account for why these results differ from those of previous studies.
First, the uneven numbers of subjects randomized to bupropion SR and placebo in the initial, double-blind portion of the study reduced the study’s statistical power. If equal numbers of patients had been placed in both treatment groups, and if the same response rates were observed, the results on the CGI-I would have achieved statistical significance. Second, the small sample size limited the overall power of the study to detect a difference. Third, it is possible that a higher dose would have produced greater differences. A study by Wilens et al (2001), with an average dose of 362 mg/day compared with our 298 mg/day, found bupropion SR to be statistically superior to placebo. However, when the bupropion SR responders in our study were compared with nonresponders at the end of the double-blind period, we found that the responders were taking slightly lower average doses (287 mg/day for responders versus 306 mg/day for the nonresponders), suggesting that a higher dose may not necessarily produce a more robust response. Fourth, given the stricter definition of improvement (compared with Wilens’ study), it is not surprising that the study had a lower percentage of medication responders. However, this study also had a higher percentage of placebo responders (22%) than other studies. A review of five controlled adult ADHD studies discovered a range of placebo responders from 4% (Spencer et al 2001) to 14% (Wilens et al 1999). The current study had four placebo responders. Three of these were followed for 4–6 months, and all three continued to do well without medication.
Finally, and perhaps most significantly, there was a very important difference between the design of this study and that of Wilens et al (2001) that might render a direct comparison misleading. All patients with current major depression, dysthymia, or symptoms of depression as measured by the HAM-D were excluded from our study. The only depressive diagnosis allowed was a single past episode of major depression that was clearly related to environmental stresses. Conversely, in Wilens’ study, 20% of the patients had a current diagnosis of major depression and 60% had past histories of major depression. Consequently, the samples of patients in these two studies may be quite different. It may be that bupropion SR works better in a depressed than a non-depressed ADHD population.
An important question in the use of bupropion in ADHD regards the appropriate duration of a trial in evaluating whether a patient will respond to the medication. In this study, responders showed a response within 4 weeks at a dose of 300 mg/day. There was little additional recruitment of bupropion responders after week 4. Conversely, in the Wilens report there were patients who seemed to become responders between weeks 4 and 6. Despite the fact that no additional responders were identified between week 4 and week 6, we would still recommend 6 weeks as the appropriate duration for short-term double-blind studies with bupropion.
There is a significant question regarding the level of improvement in ADHD symptoms required to produce clinically meaningful improvement in psychiatric adjustment. Other research groups commonly use a 30% improvement in ADHD symptoms (using different outcome measures) to define responders. In contrast, most studies on depression require a 50% improvement on the HAM-D to define responders and even higher levels to define remission. In this study, as in our previous studies, CGI-I scores of “moderately improved” were associated with 50%–60% improvements in symptoms as measured by the WRAADDS. Post hoc analyses indicated that our choice of a 50% improvement rate was as successful as the lower level in detecting a medication effect in the double-blind period. Over an extended period greater levels of symptom improvement could be expected.
In conclusion, bupropion SR was well tolerated in this study. The twice-daily dosing of bupropion is an advantage over the often more frequent dosing schedules of traditional methylphenidate. This study was limited by small sample size and its unequal distribution of patients. These findings in conjunction with previous studies suggest that bupropion SR may have a role in the treatment of adult ADHD as an alternative to stimulant medication.
Acknowledgments
Presented in part at the 40th annual meeting of the New Clinical Drug Evaluation Unit, Boca Raton, FL, USA, 30 May–2 June 2000, and at the Annual Meeting of American Psychiatric Association, Chicago, IL, USA, May 2000. Supported by GlaxoSmithKline Incorporated. The authors would like to thank Paul H Wender MD for his thoughtful analysis of this paper.
References
- Ascher JA, Cole JO, Colin JN, et al. Bupropion: a review of its mechanism of antidepressant activity. J Clin Psychiatry. 1995;56:395–401. [PubMed] [Google Scholar]
- Biederman J, Baldessarini RJ, Wright V, et al. A double-blind placebo controlled study of desipramine in the treatment of ADD: I. Efficacy. J Am Acad Child Adolesc Psychiatry. 1989;28:777–84. doi: 10.1097/00004583-198909000-00022. [DOI] [PubMed] [Google Scholar]
- Conners CK, Casat CD, Gualtieri CT, et al. Bupropion hydrochloride in attention deficit disorder with hyperactivity. J Am Acad Child Adolesc Psychiatry. 1996;35:1314–21. doi: 10.1097/00004583-199610000-00018. [DOI] [PubMed] [Google Scholar]
- Cyr M, Brown CS. Current drug therapy recommendations for the treatment of attention deficit hyperactivity disorder. Drugs. 1998;56:215–23. doi: 10.2165/00003495-199856020-00005. [DOI] [PubMed] [Google Scholar]
- Donnelly M, Zametkin AJ, Rapoport JL, et al. Treatment of childhood hyperactivity with desipramine: plasma drug concentration, cardiovascular effects, plasma and urinary catecholamine levels, and clinical response. Clin Pharmacol Ther. 1986;39:72–81. doi: 10.1038/clpt.1986.13. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Spencer TJ, et al. Attention-deficit/hyperactivity disorder in adults: an overview. Biol Psychiatry. 2000;48:9–20. doi: 10.1016/s0006-3223(00)00889-1. [DOI] [PubMed] [Google Scholar]
- Golden RN, Markey SP, Risby ED, et al. Antidepressants reduce whole-body norepinephrine turnover while enhancing 6-hydroxy-melatonin output. Arch Gen Psychiatry. 1988;45:150–4. doi: 10.1001/archpsyc.1988.01800260060008. [DOI] [PubMed] [Google Scholar]
- Heal DJ, Prow MR, Buckett WR. Measurements of 3-methoxy-4-hydroxyphenylglycol (MHPG) in mouse brain by HPLC with electrochemical detection, as an index of noradrenaline utilization and presynaptic alpha 2-adrenoreceptor function. Br J Pharmacol. 1989;96:547–56. doi: 10.1111/j.1476-5381.1989.tb11852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hechtman L. Genetic and neurobiological aspects of attention deficit hyperactive disorder: a review. J Psychiatry Neurosci. 1994;19:193–201. [PMC free article] [PubMed] [Google Scholar]
- Hedges D, Reimherr FW, Rogers A, et al. An open trial of venlafaxine in adult patients with attention deficit hyperactivity disorder. Psychopharmacol Bull. 1995;31:779–83. [PubMed] [Google Scholar]
- Huessy HR, Wright AL. The use of imipramine in children’s behavior disorders. Acta Paedopsychiatr. 1970;37:194–9. [PubMed] [Google Scholar]
- Michelson D, Adler L, Spencer T, et al. Atomoxetine in adults with ADHD: two randomized, placebo-controlled studies. Biol Psychiatry. 2003;53:112–20. doi: 10.1016/s0006-3223(02)01671-2. [DOI] [PubMed] [Google Scholar]
- Patrick KS, Caldwell RW, Ferris RM, et al. Pharmacology of the enantiomers of threo-methylphenidate. J Pharmacol Exp Ther. 1987;241:152–8. [PubMed] [Google Scholar]
- [PDR] Physicians’ Desk Reference. Montvale: Medical Economics Data Production; 2001. pp. 1489–93. [Google Scholar]
- Rapoport JL, Quinn PO, Bradbard G, et al. Imipramine and methylphenidate treatments of hyperactive boys. A double-blind comparison. Arch Gen Psychiatry. 1974;30:789–93. doi: 10.1001/archpsyc.1974.01760120049008. [DOI] [PubMed] [Google Scholar]
- Reimherr FW, Cunningham LA, Batey SR, et al. A multicenter evaluation of the efficacy and safety of 150 and 300 mg/d sustained release bupropion tablets versus placebo in depressed outpatients. Clin Ther. 1998;20:505–16. doi: 10.1016/s0149-2918(98)80060-x. [DOI] [PubMed] [Google Scholar]
- Reimherr FW, Wender PH, Marchant BK, et al. The Wender-Reimherr Adult Attention Deficit Disorder Scale as a research tool; Poster presentation at the 2003 American College of Neuro-psychopharmacology annual meeting; San Juan, Puerto Rico. 2003. [Google Scholar]
- Richelson E. Biological basis of depression and therapeutic relevance. J Clin Psychiatry. 1991;52(6 Suppl):4–10. [PubMed] [Google Scholar]
- Spencer T, Biederman J, Wilens T, et al. Nortriptyline treatment of children with attention-deficit hyperactivity disorder and tic disorder or Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry. 1993;32:205–10. doi: 10.1097/00004583-199301000-00029. [DOI] [PubMed] [Google Scholar]
- Spencer T, Biederman J, Wilens TE, et al. Effectiveness and tolerability of tomoxetine in adults with attention deficit hyperactivity disorder. Am J Psychiatry. 1998;155:693–5. doi: 10.1176/ajp.155.5.693. [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Biederman J, Wilens TE, et al. Efficacy of a mixed amphetamine salts compound in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2001;58:775–85. doi: 10.1001/archpsyc.58.8.775. [DOI] [PubMed] [Google Scholar]
- Weissman MM. The assessment of social adjustment: a review of techniques. Arch Gen Psychiatry. 1975;32:357–65. doi: 10.1001/archpsyc.1975.01760210091006. [DOI] [PubMed] [Google Scholar]
- Wender PH. Attention deficit hyperactivity disorder in adults. New York, NY: Oxford Univ Pr; 1995. [Google Scholar]
- Wender PH. Pharmacotherapy of attention-deficit/hyperactivity disorder in adults. J Clin Psychiatry. 1998;59(Suppl 7):76–9. [PubMed] [Google Scholar]
- Wender PH, Reimherr FW. Bupropion treatment of attention-deficit hyperactivity disorder in adults. Am J Psychiatry. 1990;147:1018–20. doi: 10.1176/ajp.147.8.1018. [DOI] [PubMed] [Google Scholar]
- Wender PH, Reimherr FW, Wood D, et al. A controlled study of methylphenidate in the treatment of attention deficit disorder, residual type, in adults. Am J Psychiatry. 1985;142:547–52. doi: 10.1176/ajp.142.5.547. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Biederman J, Abrantes AM, et al. A naturalistic assessment of protriptyline for attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1996;35:1485–90. doi: 10.1097/00004583-199611000-00017. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Biederman J, Spencer TJ, et al. Controlled trial of high doses of pemoline for adults with attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 1999;19:257–64. doi: 10.1097/00004714-199906000-00009. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Spencer TJ. The stimulants revisited. Child Adolesc Psychiatr Clin North Am. 2000;9:573–603. [PubMed] [Google Scholar]
- Wilens TE, Spencer TJ, Biederman J, et al. A controlled clinical trial of bupropion for attention deficit hyperactivity disorder in adults. Am J Psychiatry. 2001;158:282–8. doi: 10.1176/appi.ajp.158.2.282. [DOI] [PubMed] [Google Scholar]
- Zametkin AJ, Rapoport JL. Neurobiology of attention deficit disorder with hyperactivity: where have we come in 50 years? J Am Acad Child Adolesc Psychiatry. 1987;26:676–86. doi: 10.1097/00004583-198709000-00011. [DOI] [PubMed] [Google Scholar]


