Abstract
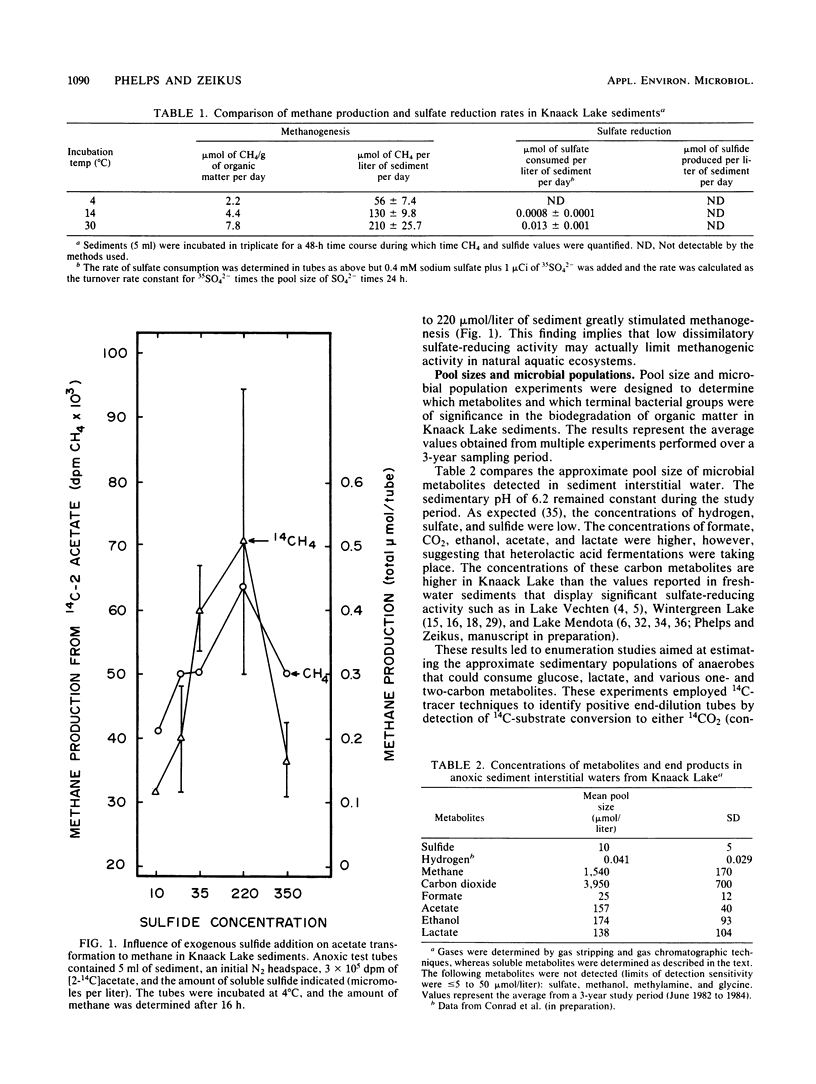
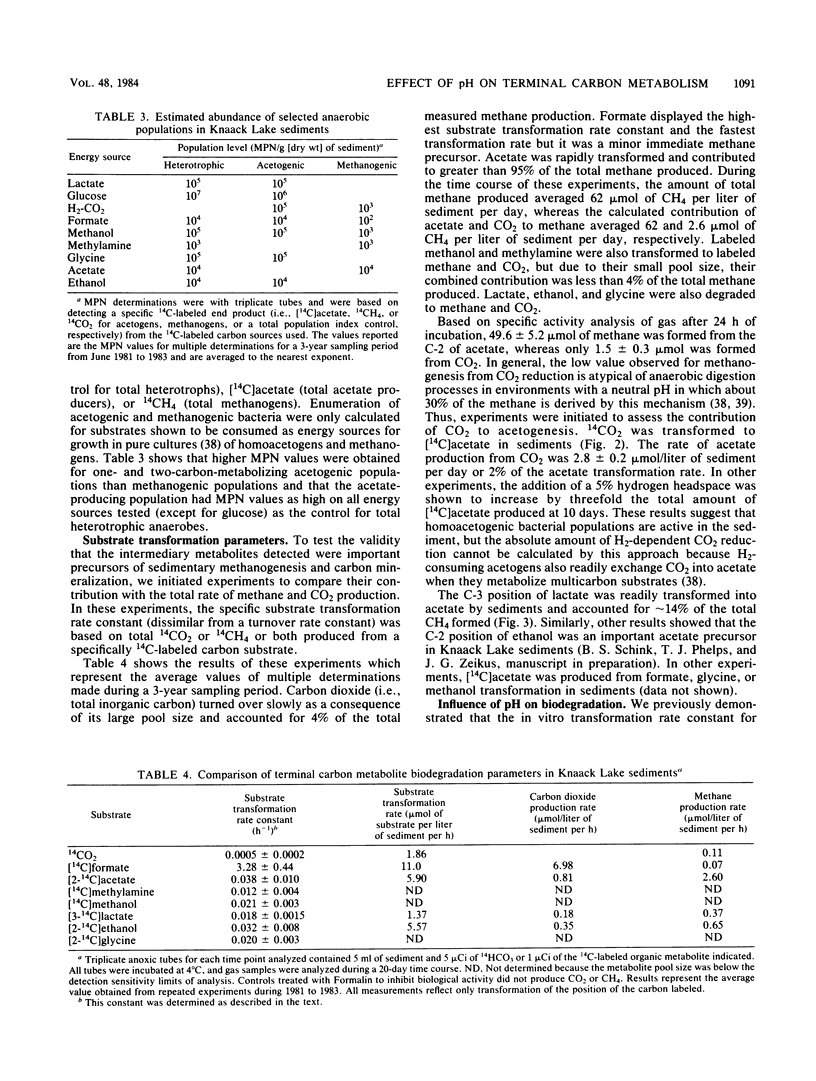
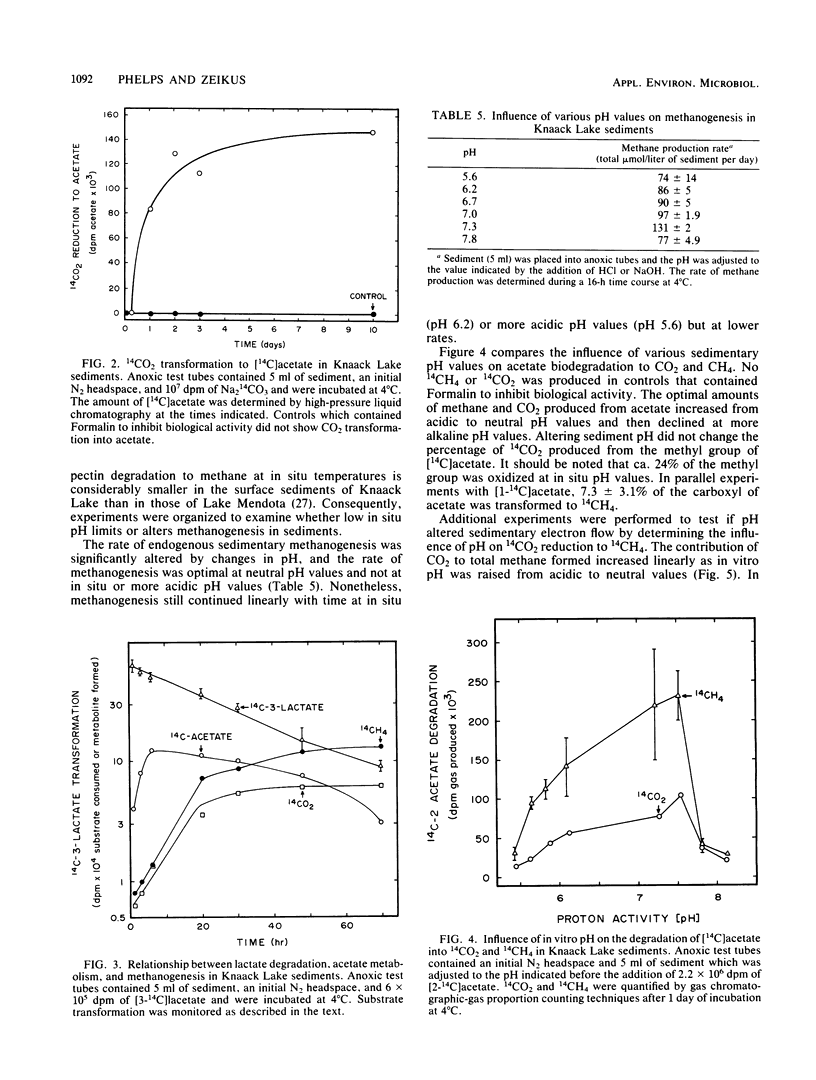
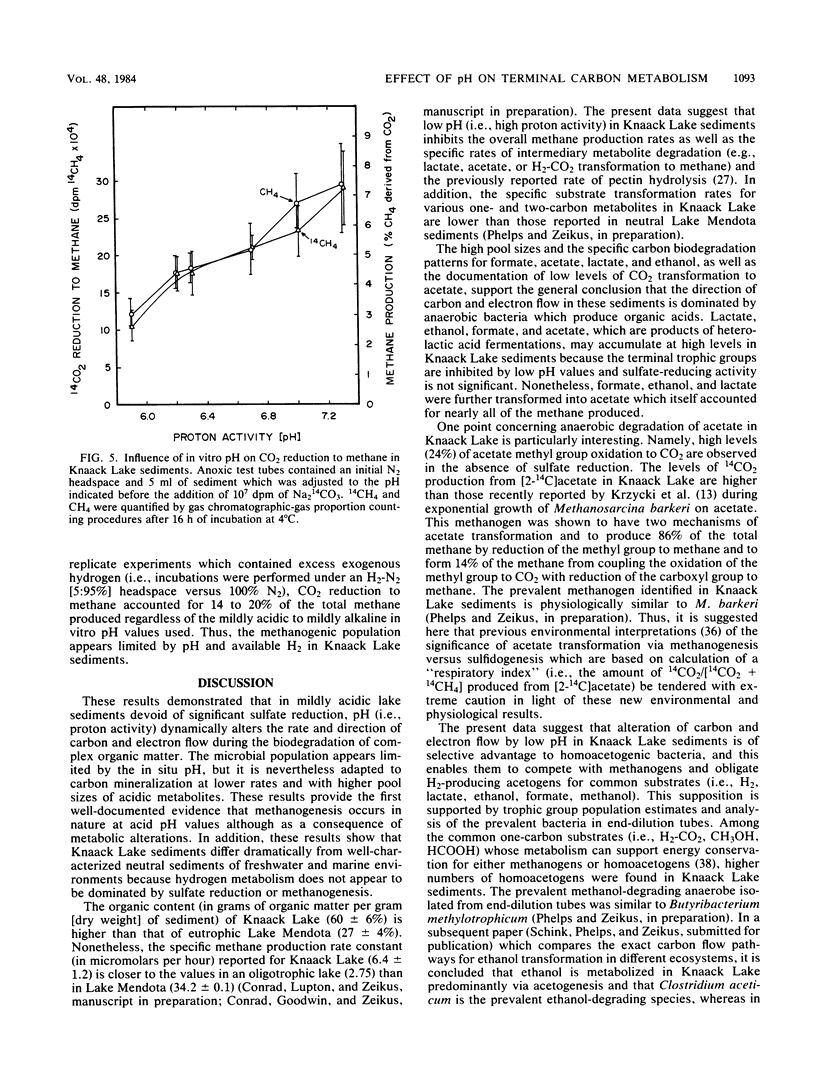
The carbon and electron flow pathways and the bacterial populations responsible for transformation of H2-CO2, formate, methanol, methylamine, acetate, glycine, ethanol, and lactate were examined in sediments collected from Knaack Lake, Wis. The sediments were 60% organic matter (pH 6.2) and did not display detectable sulfate-reducing activity, but they contained the following average concentration (in micromoles per liter of sediment) of metabolites and end products: sulfide, 10; methane, 1,540; CO2, 3,950; formate, 25; acetate, 157; ethanol, 174; and lactate, 138. Methane was produced predominately from acetate, and only 4% of the total CH4 was derived from CO2. Methanogenesis was limited by low environmental temperature and sulfide levels and more importantly by low pH. Increasing in vitro pH to neutral values enhanced total methane production rates and the percentage of CO2 transformed to methane but did not alter the amount of 14CO2 produced from [2-14C]acetate (∼24%). Analysis of both carbon transformation parameters with 14C-labeled tracers and bacterial trophic group enumerations indicated that methanogenesis from acetate and both heterolactic- and acetic acid-producing fermentations were important to the anaerobic digestion process.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abram J. W., Nedwell D. B. Inhibition of methanogenesis by sulphate reducing bacteria competing for transferred hydrogen. Arch Microbiol. 1978 Apr 27;117(1):89–92. doi: 10.1007/BF00689356. [DOI] [PubMed] [Google Scholar]
- Braun M., Schoberth S., Gottschalk G. Enumeration of bacteria forming acetate from H2 and CO2 in anaerobic habitats. Arch Microbiol. 1979 Mar 12;120(3):201–204. doi: 10.1007/BF00423066. [DOI] [PubMed] [Google Scholar]
- Cappenberg T. E. Interrelations between sulfate-reducing and methane-producing bacteria in bottom deposits of a fresh-water lake. I. Field observations. Antonie Van Leeuwenhoek. 1974;40(2):285–295. doi: 10.1007/BF00394387. [DOI] [PubMed] [Google Scholar]
- Cappenberg T. E., Prins R. A. Interrelations between sulfate-reducing and methane-producing bacteria in bottom deposits of a fresh-water lake. 3. Experiments with 14C-labeled substrates. Antonie Van Leeuwenhoek. 1974;40(3):457–469. doi: 10.1007/BF00399358. [DOI] [PubMed] [Google Scholar]
- Ingvorsen K., Zeikus J. G., Brock T. D. Dynamics of bacterial sulfate reduction in a eutrophic lake. Appl Environ Microbiol. 1981 Dec;42(6):1029–1036. doi: 10.1128/aem.42.6.1029-1036.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerby R., Niemczura W., Zeikus J. G. Single-carbon catabolism in acetogens: analysis of carbon flow in Acetobacterium woodii and Butyribacterium methylotrophicum by fermentation and 13C nuclear magnetic resonance measurement. J Bacteriol. 1983 Sep;155(3):1208–1218. doi: 10.1128/jb.155.3.1208-1218.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. M., Klug M. J., Lovley D. R. Metabolism of acetate, methanol, and methylated amines in intertidal sediments of lowes cove, maine. Appl Environ Microbiol. 1983 Jun;45(6):1848–1853. doi: 10.1128/aem.45.6.1848-1853.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch E. J., Sykes R. M. Anaerobic digestion in biological waste treatment. Prog Ind Microbiol. 1971;9:155–237. [PubMed] [Google Scholar]
- Krzycki J. A., Wolkin R. H., Zeikus J. G. Comparison of unitrophic and mixotrophic substrate metabolism by acetate-adapted strain of Methanosarcina barkeri. J Bacteriol. 1982 Jan;149(1):247–254. doi: 10.1128/jb.149.1.247-254.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovitt R. W., Longin R., Zeikus J. G. Ethanol Production by Thermophilic Bacteria: Physiological Comparison of Solvent Effects on Parent and Alcohol-Tolerant Strains of Clostridium thermohydrosulfuricum. Appl Environ Microbiol. 1984 Jul;48(1):171–177. doi: 10.1128/aem.48.1.171-177.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Dwyer D. F., Klug M. J. Kinetic analysis of competition between sulfate reducers and methanogens for hydrogen in sediments. Appl Environ Microbiol. 1982 Jun;43(6):1373–1379. doi: 10.1128/aem.43.6.1373-1379.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Klug M. J. Intermediary metabolism of organic matter in the sediments of a eutrophic lake. Appl Environ Microbiol. 1982 Mar;43(3):552–560. doi: 10.1128/aem.43.3.552-560.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Klug M. J. Methanogenesis from methanol and methylamines and acetogenesis from hydrogen and carbon dioxide in the sediments of a eutrophic lake. Appl Environ Microbiol. 1983 Apr;45(4):1310–1315. doi: 10.1128/aem.45.4.1310-1315.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Klug M. J. Sulfate reducers can outcompete methanogens at freshwater sulfate concentrations. Appl Environ Microbiol. 1983 Jan;45(1):187–192. doi: 10.1128/aem.45.1.187-192.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. R., Zeikus J. G. Rapid method for the radioisotopic analysis of gaseous end products of anaerobic metabolism. Appl Microbiol. 1974 Aug;28(2):258–261. doi: 10.1128/am.28.2.258-261.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S., Polcin S. Methanogenesis and sulfate reduction: competitive and noncompetitive substrates in estuarine sediments. Appl Environ Microbiol. 1982 Dec;44(6):1270–1276. doi: 10.1128/aem.44.6.1270-1276.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansone F. J., Martens C. S. Methane production from acetate and associated methane fluxes from anoxic coastal sediments. Science. 1981 Feb 13;211(4483):707–709. doi: 10.1126/science.211.4483.707. [DOI] [PubMed] [Google Scholar]
- Senior E., Lindström E. B., Banat I. M., Nedwell D. B. Sulfate reduction and methanogenesis in the sediment of a saltmarsh on the East coast of the United kingdom. Appl Environ Microbiol. 1982 May;43(5):987–996. doi: 10.1128/aem.43.5.987-996.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. L., Klug M. J. Electron donors utilized by sulfate-reducing bacteria in eutrophic lake sediments. Appl Environ Microbiol. 1981 Jul;42(1):116–121. doi: 10.1128/aem.42.1.116-121.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strayer R. F., Tiedje J. M. Kinetic parameters of the conversion of methane precursors to methane in a hypereutrophic lake sediment. Appl Environ Microbiol. 1978 Aug;36(2):330–340. doi: 10.1128/aem.36.2.330-340.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellinger A., Wuhrmann K. Influence of sulfide compounds on the metabolism of Methanobacterium strain AZ. Arch Microbiol. 1977 Oct 24;115(1):13–17. doi: 10.1007/BF00427839. [DOI] [PubMed] [Google Scholar]
- Winfrey M. R., Nelson D. R., Klevickis S. C., Zeikus J. G. Association of hydrogen metabolism with methanogenesis in Lake Mendota sediments. Appl Environ Microbiol. 1977 Feb;33(2):312–318. doi: 10.1128/aem.33.2.312-318.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfrey M. R., Ward D. M. Substrates for sulfate reduction and methane production in intertidal sediments. Appl Environ Microbiol. 1983 Jan;45(1):193–199. doi: 10.1128/aem.45.1.193-199.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfrey M. R., Zeikus J. G. Anaerobic metabolism of immediate methane precursors in Lake Mendota. Appl Environ Microbiol. 1979 Feb;37(2):244–253. doi: 10.1128/aem.37.2.244-253.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfrey M. R., Zeikus J. G. Effect of sulfate on carbon and electron flow during microbial methanogenesis in freshwater sediments. Appl Environ Microbiol. 1977 Feb;33(2):275–281. doi: 10.1128/aem.33.2.275-281.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfrey M. R., Zeikus J. G. Microbial methanogenesis and acetate metabolism in a meromictic lake. Appl Environ Microbiol. 1979 Feb;37(2):213–221. doi: 10.1128/aem.37.2.213-221.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G. Metabolism of one-carbon compounds by chemotrophic anaerobes. Adv Microb Physiol. 1983;24:215–299. doi: 10.1016/s0065-2911(08)60387-2. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G., Winfrey M. R. Temperature limitation of methanogenesis in aquatic sediments. Appl Environ Microbiol. 1976 Jan;31(1):99–107. doi: 10.1128/aem.31.1.99-107.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


