Abstract
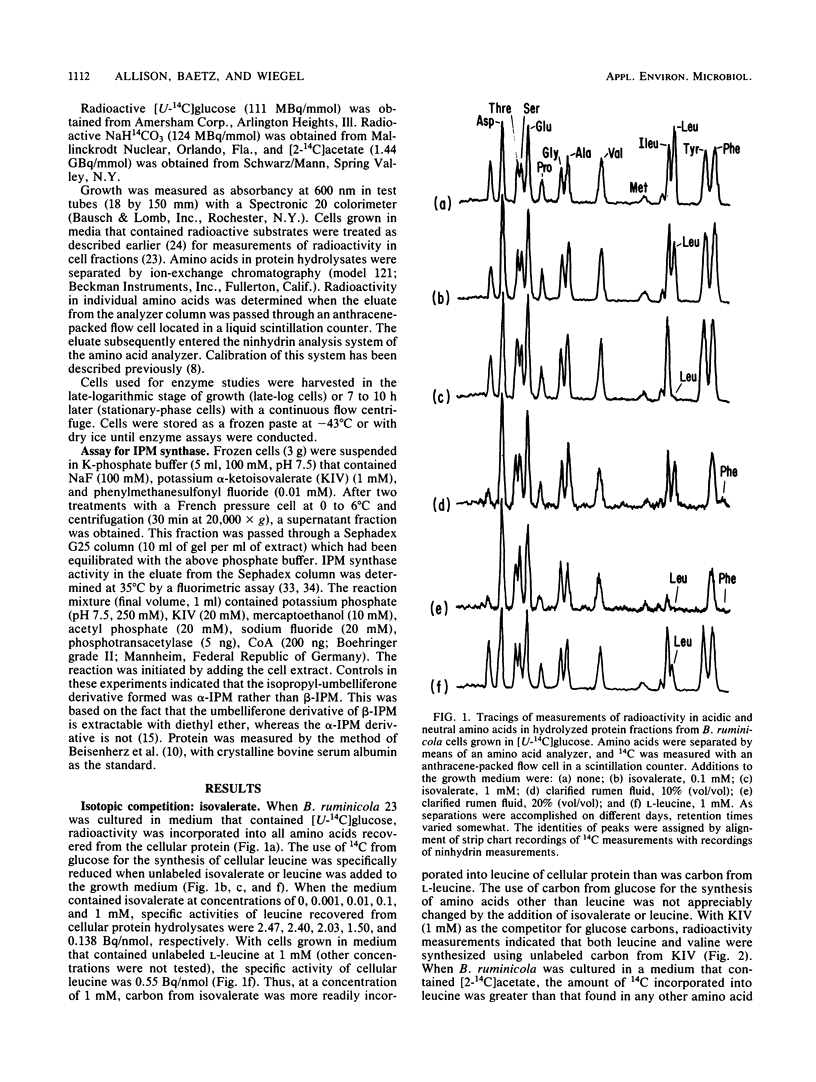
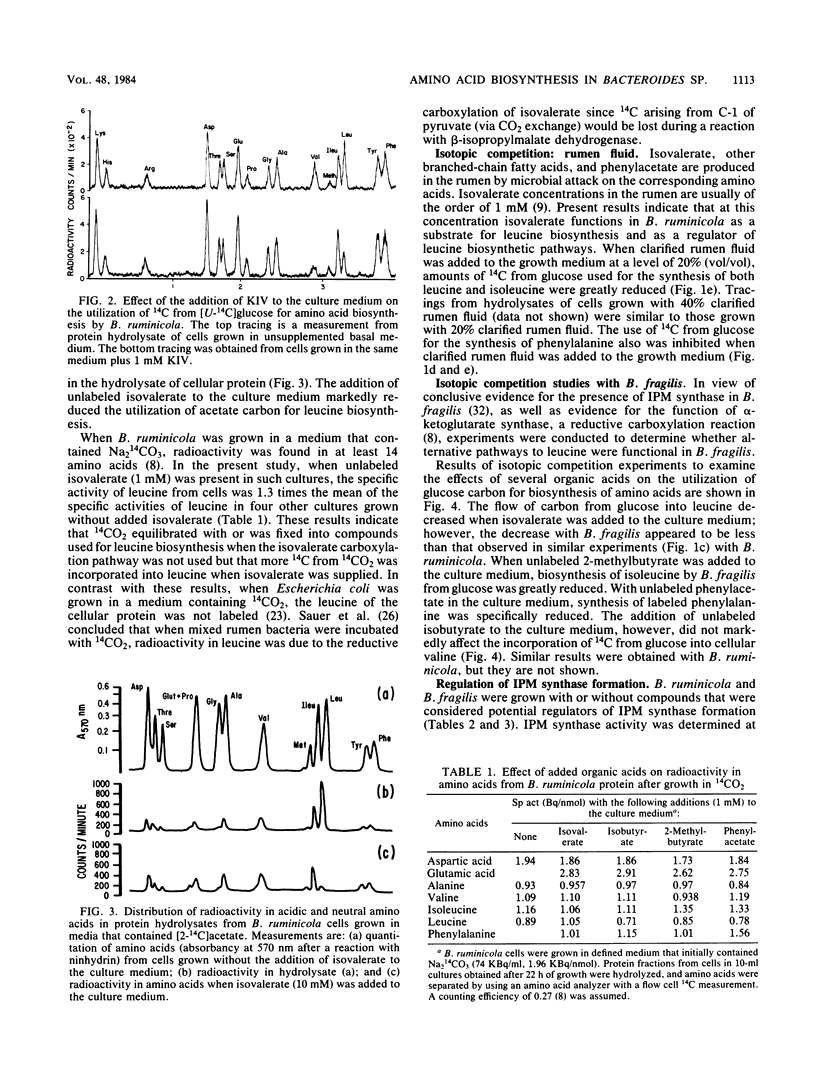
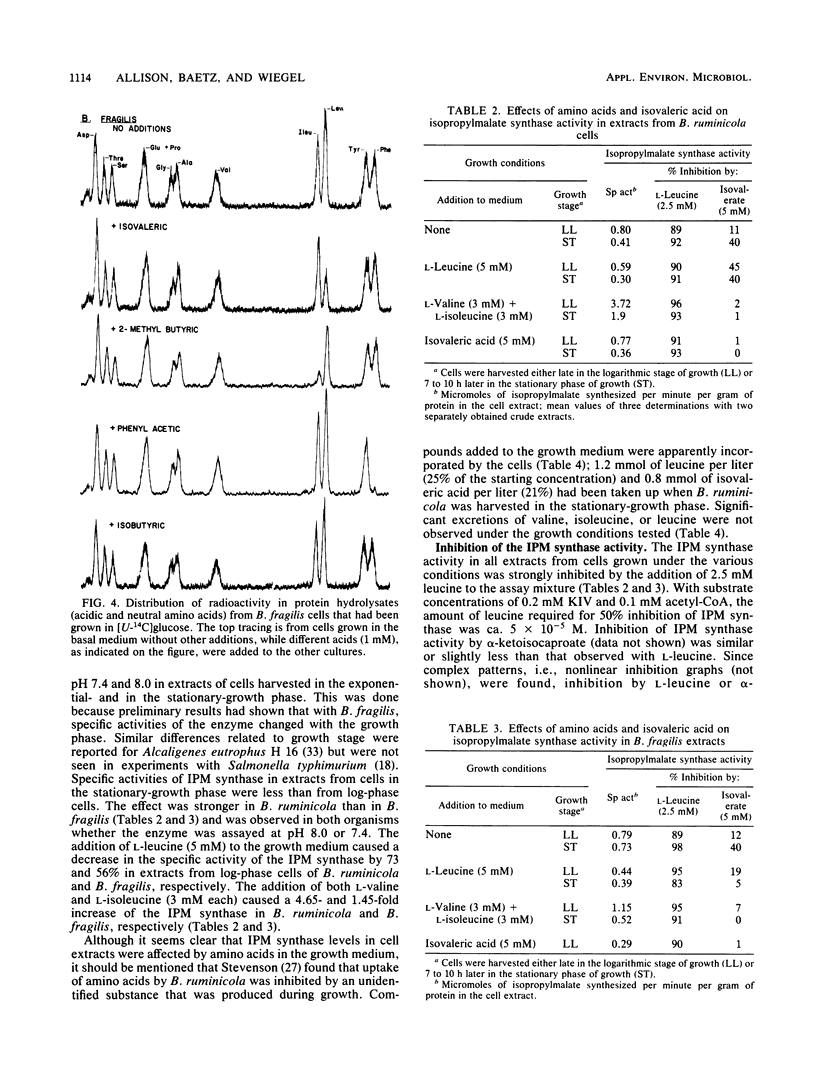
Bacteroides ruminicola is one of several species of anaerobes that are able to reductively carboxylate isovalerate (or isovaleryl-coenzyme A) to synthesize alpha-ketoisocaproate and thus leucine. When isovalerate was not supplied to growing B. ruminicola cultures, carbon from [U-14C]glucose was used for the synthesis of leucine and other cellular amino acids. When unlabeled isovalerate was available, however, utilization of [U-14C]glucose or [2-14C]acetate for leucine synthesis was markedly and specifically reduced. Enzyme assays indicated that the key enzyme of the common isopropylmalate (IPM) pathway for leucine biosynthesis, IPM synthase, was present in B. ruminicola cell extracts. The specific activity of IPM synthase was reduced when leucine was added to the growth medium but was increased by the addition of isoleucine plus valine, whereas the addition of isovalerate had little or no effect. The activity of B. ruminicola IPM synthase was strongly inhibited by leucine, the end product of the pathway. It seems unlikely that the moderate inhibition of the enzyme by isovalerate adequately explains the regulation of carbon flow by isovalerate in growing cultures. Bacteroides fragilis apparently also uses either the isovalerate carboxylation or the IPM pathway for leucine biosynthesis. Furthermore, both of these organisms synthesize isoleucine and phenylalanine, using carbon from 2-methylbutyrate and phenylacetate, respectively, in preference to synthesis of these amino acids de novo from glucose. Thus, it appears that these organisms have the ability to regulate alternative pathways for the biosynthesis of certain amino acids and that pathways involving reductive carboxylations are likely to be favored in their natural habitats.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABELSON P. H., VOGEL H. J. Amino acid biosynthesis in Torulopsis utilis and Neurospora crassa. J Biol Chem. 1955 Mar;213(1):355–364. [PubMed] [Google Scholar]
- ALLISON M. J., BRYANT M. P. Biosynthesis of branched-chain amino acids from branched-chain fatty acids by rumen bacteria. Arch Biochem Biophys. 1963 May;101:269–277. doi: 10.1016/s0003-9861(63)80012-0. [DOI] [PubMed] [Google Scholar]
- ALLISON M. J., BRYANT M. P., DOETSCH R. N. Studies on the metabolic function of branched-chain volatile fatty acids, growth factors for ruminococci. I. Incorporation of isovalerate into leucine. J Bacteriol. 1962 Mar;83:523–532. doi: 10.1128/jb.83.3.523-532.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANNISON E. F. Some observations on volatile fatty acids in the sheep's rumen. Biochem J. 1954 Jul;57(3):400–405. doi: 10.1042/bj0570400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison M. J., Bucklin J. A., Robinson I. M. Importance of the isovalerate carboxylation pathway of leucine biosynthesis in the rumen. Appl Microbiol. 1966 Sep;14(5):807–814. doi: 10.1128/am.14.5.807-814.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison M. J., Peel J. L. The biosynthesis of valine from isobutyrate by peptostreptococcus elsdenii and Bacteroides ruminicola. Biochem J. 1971 Feb;121(3):431–437. doi: 10.1042/bj1210431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison M. J. Production of branched-chain volatile fatty acids by certain anaerobic bacteria. Appl Environ Microbiol. 1978 May;35(5):872–877. doi: 10.1128/aem.35.5.872-877.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison M. J., Robinson I. M., Baetz A. L. Synthesis of alpha-ketoglutarate by reductive carboxylation of succinate in Veillonella, Selenomonas, and Bacteriodes species. J Bacteriol. 1979 Dec;140(3):980–986. doi: 10.1128/jb.140.3.980-986.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison M. J., Robinson I. M. Biosynthesis of alpha-ketoglutarate by the reductive carboxylation of succinate in Bacteroides ruminicola. J Bacteriol. 1970 Oct;104(1):50–56. doi: 10.1128/jb.104.1.50-56.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRYANT M. P., SMALL N., BOUMA C., CHU H. Bacteroides ruminicola n. sp. and Succinimonas amylolytica; the new genus and species; species of succinic acid-producing anaerobic bacteria of the bovine rumen. J Bacteriol. 1958 Jul;76(1):15–23. doi: 10.1128/jb.76.1.15-23.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P., Robinson I. M. Some Nutritional Requirements of the Genus Ruminococcus. Appl Microbiol. 1961 Mar;9(2):91–95. doi: 10.1128/am.9.2.91-95.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns R. O., Calvo J., Margolin P., Umbarger H. E. Expression of the leucine operon. J Bacteriol. 1966 Apr;91(4):1570–1576. doi: 10.1128/jb.91.4.1570-1576.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhaw G., Leary T. R., Umbarger H. E. Alpha-isopropylmalate synthase from Salmonella typhimurium. Purification and properties. J Biol Chem. 1969 Apr 25;244(8):2218–2225. [PubMed] [Google Scholar]
- Monticello D. J., Hadioetomo R. S., Costilow R. N. Isoleucine synthesis by Clostridium sporogenes from propionate or alpha-methylbutyrate. J Gen Microbiol. 1984 Feb;130(2):309–318. doi: 10.1099/00221287-130-2-309. [DOI] [PubMed] [Google Scholar]
- Patel N., Pierson D. L., Jensen R. A. Dual enzymatic routes to L-tyrosine and L-phenylalanine via pretyrosine in Pseudomonas aeruginosa. J Biol Chem. 1977 Aug 25;252(16):5839–5846. [PubMed] [Google Scholar]
- Phillips A. T., Nuss J. I., Moosic J., Foshay C. Alternate pathway for isoleucine biosynthesis in Escherichia coli. J Bacteriol. 1972 Feb;109(2):714–719. doi: 10.1128/jb.109.2.714-719.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson I. M., Allison M. J. Isoleucine biosynthesis from 2-methylbutyric acid by anaerobic bacteria from the rumen. J Bacteriol. 1969 Mar;97(3):1220–1226. doi: 10.1128/jb.97.3.1220-1226.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyanarayana T., Umbarger H. E., Lindegren G. Biosynthesis of branched-chain amino acids in yeast: regulation of leucine biosynthesis in prototrophic and leucine auxotrophic strains. J Bacteriol. 1968 Dec;96(6):2018–2024. doi: 10.1128/jb.96.6.2018-2024.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer F. D., Erfle J. D., Mahadevan S. Amino acid biosynthesis in mixed rumen cultures. Biochem J. 1975 Sep;150(3):357–372. doi: 10.1042/bj1500357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson R. M. Amino acid uptake systems in Bacteroides ruminicola. Can J Microbiol. 1979 Oct;25(10):1161–1168. doi: 10.1139/m79-180. [DOI] [PubMed] [Google Scholar]
- Stieglitz B. I., Calvo J. M. Distribution of the isopropylmalate pathway to leucine among diverse bacteria. J Bacteriol. 1974 Jun;118(3):935–941. doi: 10.1128/jb.118.3.935-941.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbarger H. E. Amino acid biosynthesis and its regulation. Annu Rev Biochem. 1978;47:532–606. doi: 10.1146/annurev.bi.47.070178.002533. [DOI] [PubMed] [Google Scholar]
- Westfall H. N., Charon N. W., Peterson D. E. Multiple pathways for isoleucine biosynthesis in the spirochete Leptospira. J Bacteriol. 1983 May;154(2):846–853. doi: 10.1128/jb.154.2.846-853.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegel J. Alpha-isopropylmalate synthase as a marker for the leucine biosynthetic pathway in several clostridia and in Bacteroides fragilis. Arch Microbiol. 1981 Dec 2;130(5):385–390. doi: 10.1007/BF00414605. [DOI] [PubMed] [Google Scholar]
- Wiegel J., Schlegel H. G. Alpha-Isopropylmalate synthase from Alcaligenes eutrophus H 16 I. Purification and general properties. Arch Microbiol. 1977 Apr 1;112(3):239–246. doi: 10.1007/BF00413087. [DOI] [PubMed] [Google Scholar]
- Wiegel J., Schlegel H. G. alpha-Isopropylmalate synthase from Alcaligenes eutrophus H 16. II. Substrate specificity and kinetics. Arch Microbiol. 1977 Apr 1;112(3):247–254. doi: 10.1007/BF00413088. [DOI] [PubMed] [Google Scholar]


