Abstract
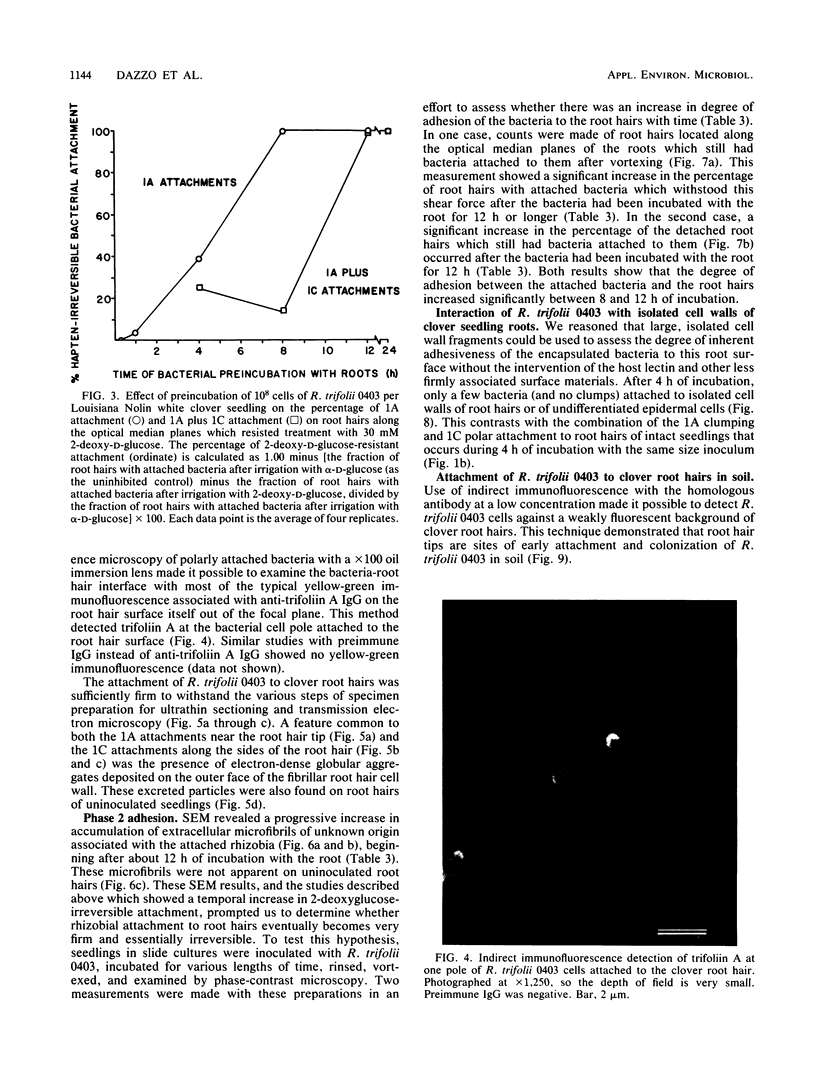
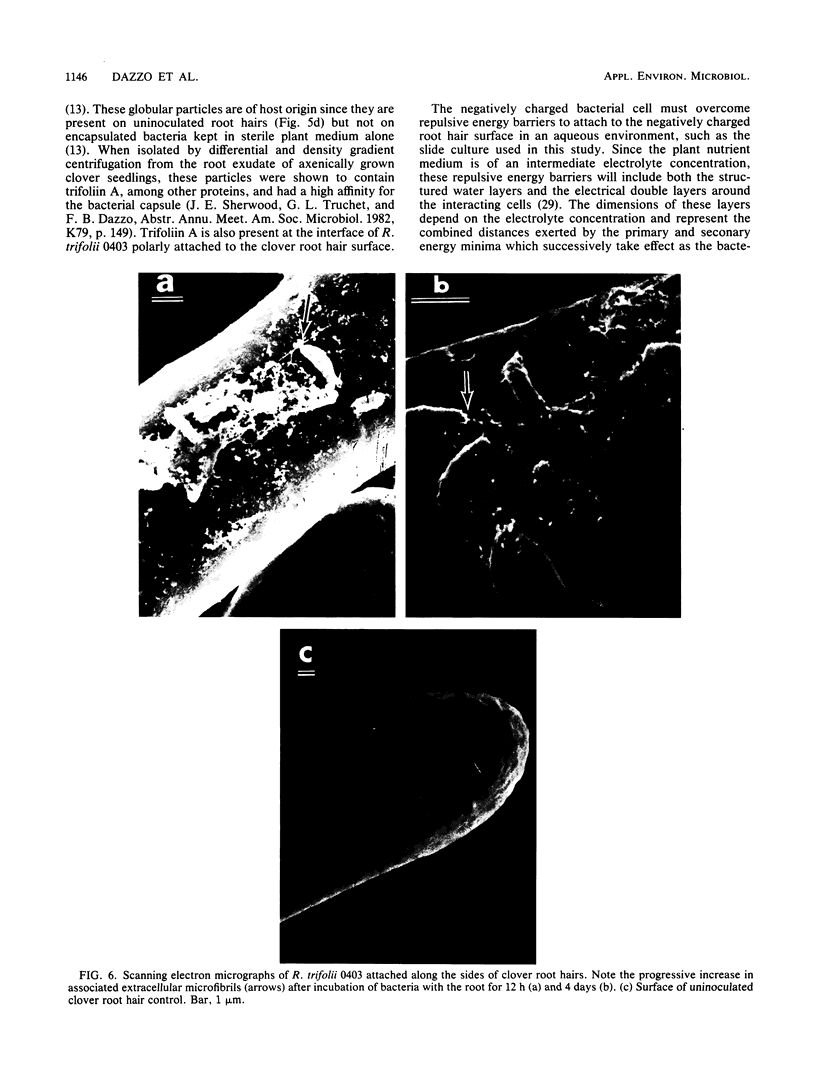
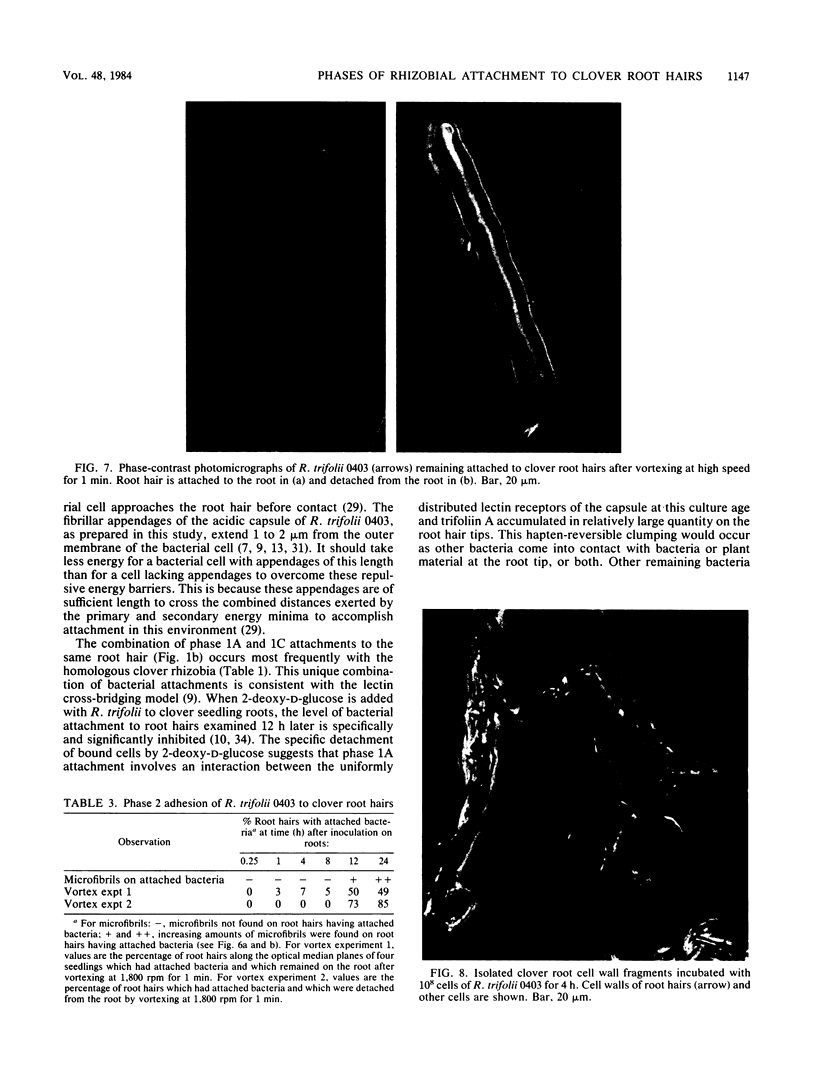
The time course and orientation of attachment of Rhizobium trifolii 0403 to white clover root hairs was examined in slide cultures by light and electron microscopy. Inocula were grown for 5 days on defined BIII agar medium and represented the large subpopulation of fully encapsulated single cells which uniformly bind the clover lectin trifoliin A. When 10(7) cells or more were added per seedling, bacteria attached within minutes, forming randomly oriented clumps at the root hair tips. Several hours later, single cells attached polarly to the sides of the root hair. This sequence of attachment to clover root hairs was selective for R. trifolii at inoculum sizes of 10(7) to 4 X 10(8) per seedling, specifically inhibited if 2-deoxy-D-glucose, a hapten for trifoliin A, was present in the inoculum, and not observed when 4 X 10(8) cells were added to alfalfa seedling roots or to large clover root cell wall fragments which lacked trifoliin A but still had trifoliin A receptors. Once attached, R. trifolii 0403 became progressively less detachable with 2-deoxy-D-glucose. At smaller inoculum sizes (10(5) to 10(6) cells per seedling), there was no immediate clumping of R. trifolii at clover root hair tips, although polar binding of bacteria along the root hair surface was observed after 4 h. The interface between polarly attached bacteria and the root hair cell wall was shown to contain trifoliin A by immunofluorescence microscopy. Also, this interface was shown by transmission electron microscopy to contain electron-dense granules of host origin. Scanning electron microscopy revealed an accumulation of extracellular microfibrils associated with the lateral and polar surfaces of the attached bacteria, detectable after 12 h of incubation with seedling roots. At this same time, there was a significant reduction in the effectiveness of 2-deoxy-D-glucose in dislodging bacteria already attached to root hairs and an increase in firm attachment of bacteria to the root hair surface, which withstood the hydrodynamic shear forces of high-speed vortexing. These results are interpreted as a sequence of phases in attachment, beginning with specific reversible interactions between bacterial and plant surfaces (phase I attachment), followed by production of extracellular microfibrils which firmly anchor the bacterium to the root hair (phase 2 adhesion). Thus, attachment of R. trifolii to clover root hairs is a specific process requiring more than just the inherent adhesiveness of the bacteria to the plant cell wall.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop P. E., Dazzo F. B., Appelbaum E. R., Maier R. J., Brill W. J. Intergeneric transfer of genes involved in the Rhizobium-legume symbiosis. Science. 1977 Dec 2;198(4320):938–940. doi: 10.1126/science.929179. [DOI] [PubMed] [Google Scholar]
- Dazzo F. B., Brill W. J. Bacterial polysaccharide which binds Rhizobium trifolii to clover root hairs. J Bacteriol. 1979 Mar;137(3):1362–1373. doi: 10.1128/jb.137.3.1362-1373.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Hubbell D. H. Cross-reactive antigens and lectin as determinants of symbiotic specificity in the Rhizobium-clover association. Appl Microbiol. 1975 Dec;30(6):1017–1033. doi: 10.1128/am.30.6.1017-1033.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Napoli C. A., Hubbell D. H. Adsorption of bacteria to roots as related to host specificity in the Rhizobium-clover symbiosis. Appl Environ Microbiol. 1976 Jul;32(1):166–171. doi: 10.1128/aem.32.1.166-171.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Truchet G. L., Sherwood J. E., Hrabak E. M., Gardiol A. E. Alteration of the Trifoliin A-Binding Capsule of Rhizobium trifolii 0403 by Enzymes Released from Clover Roots. Appl Environ Microbiol. 1982 Aug;44(2):478–490. doi: 10.1128/aem.44.2.478-490.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Yanke W. E., Brill W. J. Trifolin: a Rhizobium recognition protein from white clover. Biochim Biophys Acta. 1978 Mar 20;539(3):276–286. doi: 10.1016/0304-4165(78)90032-6. [DOI] [PubMed] [Google Scholar]
- Deinema M. H., Zevenhuizen L. P. Formation of cellulose fibrils by gram-negative bacteria and their role in bacterial flocculation. Arch Mikrobiol. 1971;78(1):42–51. doi: 10.1007/BF00409087. [DOI] [PubMed] [Google Scholar]
- Higashi S., Abe M. Scanning Electron Microscopy of Rhizobium trifolii Infection Sites on Root Hairs of White Clover. Appl Environ Microbiol. 1980 Dec;40(6):1094–1099. doi: 10.1128/aem.40.6.1094-1099.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Richardson L. A., Uhlman D. The invasion of HeLa cells by Salmonella typhimurium: reversible and irreversible bacterial attachment and the role of bacterial motility. J Gen Microbiol. 1981 Dec;127(2):351–360. doi: 10.1099/00221287-127-2-351. [DOI] [PubMed] [Google Scholar]
- Marshall K. C., Cruickshank R. H., Bushby H. V. The orientation of certain root-nodule bacteria at interfaces, including legume root-hair surfaces. J Gen Microbiol. 1975 Nov;91(1):198–200. doi: 10.1099/00221287-91-1-198. [DOI] [PubMed] [Google Scholar]
- Matthysse A. G., Holmes K. V., Gurlitz R. H. Elaboration of cellulose fibrils by Agrobacterium tumefaciens during attachment to carrot cells. J Bacteriol. 1981 Jan;145(1):583–595. doi: 10.1128/jb.145.1.583-595.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel G., Uhlig H., Weichsel G. Uber die Besiedlung der Wurzeln einiger Leguminosen und Nichtleguminosen mit Rhizobien und anderen Bodenbakterien. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg. 1972;127(4):348–358. [PubMed] [Google Scholar]
- Moore R. L., Marshall K. C. Attachment and rosette formation by hyphomicrobia. Appl Environ Microbiol. 1981 Nov;42(5):751–757. doi: 10.1128/aem.42.5.751-757.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli C., Dazzo F., Hubbell D. Production of cellulose microfibrils by Rhizobium. Appl Microbiol. 1975 Jul;30(1):123–131. doi: 10.1128/am.30.1.123-131.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paau A. S., Leps W. T., Brill W. J. Agglutinin from Alfalfa Necessary for Binding and Nodulation by Rhizobium meliloti. Science. 1981 Sep 25;213(4515):1513–1515. doi: 10.1126/science.213.4515.1513. [DOI] [PubMed] [Google Scholar]
- SAHLMAN K. AN ELECTRON MICROSCOPE STUDY OF ROOT-HAIR INFECTION BY RHIZOBIUM. J Gen Microbiol. 1963 Dec;33:425–427. doi: 10.1099/00221287-33-3-425. [DOI] [PubMed] [Google Scholar]
- Sherwood J. E., Vasse J. M., Dazzo F. B., Truchet G. L. Development and trifoliin A-binding ability of the capsule of Rhizobium trifolii. J Bacteriol. 1984 Jul;159(1):145–152. doi: 10.1128/jb.159.1.145-152.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey G., Paau A. S., Brill W. J. Host recognition in the Rhizobium-soybean symbiosis. Plant Physiol. 1980 Oct;66(4):609–614. doi: 10.1104/pp.66.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurkowski W. Specific adsorption of bacteria to clover root hairs, related to the presence of the plasmid pWZ2 in cells of Rhizobium trifolii. Microbios. 1980;27(107):27–32. [PubMed] [Google Scholar]
- van Rensburg H. J., Strijdom B. W. Root Surface Association in Relation to Nodulation of Medicago sativa. Appl Environ Microbiol. 1982 Jul;44(1):93–97. doi: 10.1128/aem.44.1.93-97.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]











