Abstract
Analysis of aged and cataract lenses shows the presence of increased amounts of crystallin fragments in the high molecular weight aggregates of water-soluble and water-insoluble fractions. However, the significance of accumulation and interaction of low molecular weight crystallin fragments in aging and cataract development is not clearly understood. In this study, 23 low molecular mass (<3.5-kDa) peptides in the urea-soluble fractions of young, aged, and aged cataract human lenses were identified by mass spectroscopy. Two peptides, αB-(1–18) (MDIAIHHPWIRRPFFPFH) and βA3/A1-(59–74) (SD(N)AYHIERLMSFRPIC), present in aged and cataract lens but not young lens, and a third peptide, γS-(167–178) (SPAVQSFRRIVE) present in all three lens groups were synthesized to study the effects of interaction of these peptides with intact α-, β-, and γ-crystallins and alcohol dehydrogenase, a protein used in aggregation studies. Interaction of αB-(1–18) and βA3/A1-(59–74) peptides increased the scattering of light by β- and γ-crystallin and alcohol dehydrogenase. The ability of α-crystallin subunits to function as molecular chaperones was significantly reduced by interaction with αB-(1–18) and βA3/A1-(59–74) peptides, whereas γS peptide had no effect on chaperone-like activity of α-crystallin. The βA3/A1-(59–74 peptide caused a 5.64-fold increase in αB-crystallin oligomeric mass and partial precipitation. Replacing hydrophobic residues in αB-(1–18) and βA3/A1-(59–74) peptides abolished their ability to induce crystallin aggregation and light scattering. Our study suggests that interaction of crystallin-derived peptides with intact crystallins could be a key event in age-related protein aggregation in lens and cataractogenesis.
Lens proteins undergo very little turnover after synthesis, but they undergo various changes during aging and cataractogenesis (1). Certain post-translational modifications appear to be part of the normal maturation process (2, 3), and other modifications, including deamidation, phosphorylation, truncation, glycation, oxidation, and cross-linking, are seen in aged and cataractous lenses (4). Changes in the structure and solubility of lens proteins depend on the extent and nature of the modification. Lens opacification is primarily due to the insolubilization of crystallins, proteins that are essential for maintaining the optical properties of the lens.
Crystallin proteolysis is significantly increased in aged and cataract human lenses as compared with young human lenses (5–8). Increased crystallin fragmentation in aged and cataract lenses has also been reported in nonhuman species (3, 9–12). Unregulated proteolysis of crystallins is suggested to be one of the cause for crystallin insolubilization (13). Several proteolytic enzymes have been shown to play a role in aging of the lens and cataract formation (14–20). Degradation of lens crystallins by proteolytic enzymes can damage their highly ordered arrangements. An age-related decrease in the levels or activities of proteases could result in the accumulation of modified proteins (21, 22). Although a protein oxidation reaction may convert proteins to forms that are more susceptible to proteolytic degradation, the oxidized forms of some proteins not only are resistant to proteolytic degradation but also may inhibit the ability of some proteases to degrade the oxidized forms of other proteins. The protein-protein cross-linked derivatives have been found to resist proteolytic degradation and to inhibit the ability of the 20 S proteosome to degrade the oxidized forms of other proteins (23).
Crystallin fragments are present in water-soluble and water-insoluble factions of the lens, with more prevalence in the water-insoluble fractions (6, 24, 25). As many as 13 crystallin fragments with molecular masses between 3 and 17 kDa originating from α-, β-, and γ-crystallins have been isolated from the water-soluble high molecular weight aggregates of 60–80-year-old human lens (26). Understanding the interaction between the lens proteins and the crystallin fragments is the key to assessing the consequence of incomplete proteolytic degradation. Protein aggregation in the lens increases with age, leading to the accumulation of high molecular weight aggregates that scatter light. Although it is hypothesized that cataract develops as a result of the improper interaction of crystallin fragments generated by proteolysis (17), the mechanism by which crystallin fragments initiate or influence the process of lens protein aggregation to form high molecular weight aggregates is not clear. Earlier, we showed that in vitro oxidized crystallin peptides enhance the aggregation of βL-crystallin and γ-crystallin and also exhibit an anti-chaperone-like property (27–29). Peptide interactions with lens proteins and the aggregation-facilitating nature of lens peptides may be important in age-related cataract formation, where lens proteins undergo incomplete proteolysis and peptide fragments tend to accumulate.
The present study was performed to identify crystallin fragments in young, aged, and cataract human lenses and investigate their possible role in crystallin aggregation and light scattering. Our data show that peptides generated in the lens interact with crystallins and increase their aggregation and precipitation. The results also demonstrate that crystallin peptides generated in vivo exhibit anti-chaperone-like activity.
EXPERIMENTAL PROCEDURES
Materials—αB-crystallin peptide (1MDIAIHHPWIRRPFFPFH18) (peptide A), βA3/A1-crystallin peptide (59SD(N)AYHIERLMSFRPIC74) (peptide B), and γS-crystallin peptide (167SPAVQSFRRIVE178) (peptide G) and the mutant peptides were synthesized at the University of Missouri-Columbia core facility. The protease inhibitor mixture was obtained from Calbiochem. Alcohol dehydrogenase (ADH)2 was obtained from Biozyme Laboratories (San Diego, CA). All other chemicals were of analytical grade and from Sigma unless otherwise noted.
Lenses—Human donor lenses were obtained from the Lions Eye Tissue Bank (Columbia, MO) and kept at -70 °C until use. Lenses were separated based on their age and color. Four lenses were analyzed from each group: young lenses (18–32 years), aged lenses (75–95 years), and aged cataract lenses. Young (2 years old) bovine lenses were obtained from Pel-Freeze Biologicals (Rogers, AR) and stored at -70 °C until use.
Isolation and Identification of Peptides from Human Lens—Lenses were homogenized in 1 ml of 0.05 m phosphate buffer (pH 7.4) containing 0.1 m NaCl, 6 m urea, and 2 mm tris(2-carboxyethyl)phosphine hydrochloride (Pierce) and in the presence of 5 μl of protease inhibitor mixture. The sample was centrifuged at 16,000 × g for 1 h. The urea-soluble fraction was collected and filtered through a 10 kDa Centriplus filter (Millipore). The low molecular weight (LMW) peptides that were present in the filtrate were desalted using PepClean C-18 spin columns (Pierce). The bound peptides were eluted by 70% acetonitrile containing 1% formic acid. The eluted peptides were analyzed by ABI Voyager DE PRO matrix-assisted laser desorption time of flight mass spectrometry (MALDI-TOF MS) and nanoelectrospray ionization quadrupole time-of-flight tandem mass spectrometry to identify and sequence the peptide ions.
The low molecular weight fractions prepared from young, old, and cataract human lenses as above was dialyzed extensively using 1-kDa cut-off dialysis tubing, concentrated, and used in quantifying the relative light scattering activity in an aggregation assay.
Preparation of Peptides—The synthetic peptides obtained from the core facility were purified by reverse-phase high performance liquid chromatography on a C-18 column. The peptide mass and sequences were confirmed by mass spectrometric analysis at the University of Missouri-Columbia Proteomics Center. The purified peptides were dissolved in a small volume of Me2SO and equilibrated with 0.05 m phosphate buffer containing 0.15 m NaCl (pH 7.4) using 1-kDa dialysis membrane. The peptide concentration in the dialyzed sample was measured using a micro-BCA protein assay kit (Pierce).
Regional Distribution of LMW Peptides—A pair of 75-year-old lenses was used to measure the peptide concentration in the cortex and nucleus of aged human lens. The lens was thawed, and the capsule was carefully removed using a forceps. The outer cortex and inner nucleus were separated using the microstir bar in a glass vial using 0.05 m phosphate buffer containing 6 m urea, 2 mm tris(2-carboxyethyl)phosphine hydrochloride and protease inhibitor mixture (20 μl/5 ml of buffer), pH 7.4. The lenses were stirred for about 3 min in 0.5 ml of buffer to solubilize the cortex. The nucleus was homogenized using a micro-tissue homogenizer using the same buffer. The sample volume was adjusted to 1.5 ml with homogenization buffer and centrifuged at 13,000 × g to remove the urea-insoluble fraction. 0.5 ml of the supernatant from the cortex and nucleus fraction was passed through 10 kDa cut-off filter to obtain the LMW peptides. 30 μl of the filtrate containing LMW peptides was desalted using PepClean spin column and analyzed by MALDI-TOF MS.
Purification of Crystallins—α-Crystallin, β-crystallin, and γ-crystallin were separated from the water-soluble fractions of young bovine lens cortex by gel filtration on a Sephadex G-200 column (30). α-Crystallin was further purified on a trimethyl-aminoethyl fractogel ion exchange column, as described previously (29). βL-Crystallin peak fractions were pooled, concentrated, and used without further purification. γ-Crystallin was isolated from bovine lens extract by means of Sephadex G-200 and Biogel P-30 chromatography, as described previously (31). The protein concentration was measured using a BCA protein assay kit. Recombinant human αA- and αB-crystallins were expressed in Escherichia coli BL21(DE3)pLysS cells (Invitrogen) and purified using gel filtration and anion exchange chromatography, as described previously (32).
Interaction between Crystallin Peptides and Intact Crystallins—Interactions between intact α-, β-, and γ-crystallins and crystallin peptides A, B, and G were verified using HPLC and SDS-PAGE analysis.
Recombinant αA-crystallin (250 μg) was incubated with 100 μg of peptide A, B, or G, in a total volume of 0.5 ml of 50 mm phosphate buffer containing 150 mm NaCl (pH 7.4) at 37 °C. Samples that contained αA-crystallin or peptide alone served as control. After 4 h of incubation, the samples were briefly centrifuged to separate any visible precipitate. Supernatant (350 μl) was injected into a TSK G5000PWXL (Tosoh Bioscience, Montgomeryville, PA) size exclusion column fitted to an HPLC system with a UV detector and equilibrated with phosphate buffer. The peak containing the αA-crystallin-peptide complex was pooled, concentrated, and analyzed on 4–20% Tris-HCl SDS-polyacrylamide gel (Bio-Rad) electrophoresis.
The interactions of βL- and γ-crystallin with each peptide were assessed by incubating 250 μg of the crystallin with 100 μg of the peptide at 55 °C for 1 h. Crystallin by itself was used as a control. At the end of incubation, the aggregated protein was collected by centrifugation at 15,000 × g. The precipitated protein was washed with deionized water and centrifuged to remove any soluble proteins or peptides. The procedure was repeated twice. The collected precipitate was solubilized in 200 μl of freshly prepared 6 m urea and diluted with water until the sample measured 1 ml. The sample was injected onto a reverse-phase analytical C-18 column (Vydac) equilibrated with water containing 0.1% trifluoroacetic acid. The βL-crystallin subunits and the peptides were separated using acetonitrile gradient (5–80%).
Light Scattering Measurements—The effects of peptide interaction on the molecular mass and the hydrodynamic radius of αB-crystallin were studied using static and dynamic light scattering. Recombinant αB-crystallin (300 μg) was incubated at 37 °C for 4 h with an equal amount (weight) of the peptide in 0.5 ml of 0.05 m phosphate buffer (pH 7.2) containing 0.15 m NaCl. At the end of incubation, the samples were centrifuged to remove visible precipitates. Supernatant (150 μl) was injected into a TSK G5000PWXL (Tosoh Bioscience) size exclusion column fitted to a high pressure liquid chromatograph with a refractive index detector (Shimadzu, Columbia, MD) and equilibrated with the incubation buffer. The HPLC was coupled to multiangle light scattering and quasielastic light scattering detectors (Wyatt Technology Corp., Santa Barbara, CA). Data were analyzed using ASTRA (5.1.9) software developed by Wyatt Technology.
Thermal Denaturation and Light Scattering Assay—Thermal aggregation assay of βL-crystallin, γ-crystallin, and ADH and chaperone assay of α-crystallin were performed according to the method described previously (29, 32). Briefly, substrate protein βL-crystallin (100 μg) or γ-crystallin (100 μg) was heat-denatured at 55 °C in 1 ml of 0.05 m phosphate buffer, pH 7.4. Aggregation and light scattering were monitored at 360 nm. Different concentrations of peptide A, peptide B, and peptide G were included in the assay mixture to determine their effect on substrate protein aggregation. A similar experiment was performed following incubation at 37 °C for 10 h to study the effect of each peptide on βL-crystallin aggregation at physiological temperature. To study the anti-chaperone activity of low molecular weight peptides, known concentrations of peptides A, B, and G were included in the assay mixture along with chaperone protein, α-crystallin, and substrate protein. Aggregation and light scattering were monitored as described above. To determine the specificity of these peptides in influencing protein aggregation, a few mutants of peptide A and peptide B were used to study their effects on protein aggregation and light scattering. ADH (250 μg) was chosen as a noncrystallin substrate protein to study the interaction with crystallin fragments.
RESULTS
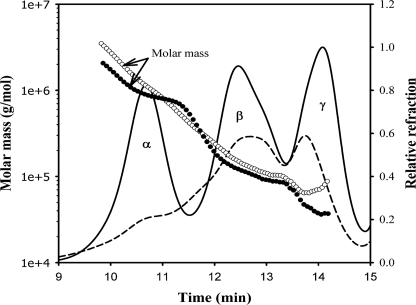
Water-soluble protein fractions from young and aged human lenses were subjected to size exclusion chromatography coupled with dynamic light scattering analysis. We observed that, compared with the water-soluble fraction of young lens, the protein concentration in the water-soluble fraction of aged lens was decreased, whereas the mass distribution was increased (Fig. 1). The aged lens had soluble aggregates as large as 3500 kDa in contrast to soluble aggregates of 2000 kDa in the young lens.
FIGURE 1.
Mass distribution in the water-soluble fraction in young (19 years) (solid line and filled circle) and aged (83 years) (broken line and open circle) human lenses using multiangle light scattering. The samples (250 μg of proteins) were injected into a high pressure liquid chromatograph fitted with a TSK G5000PWXL size exclusion column and refractive index detector, coupled with multiangle light scattering and quasielastic light scattering detectors. The mobile phase contained 0.05 m sodium phosphate and 0.15 m sodium chloride, pH 7.2. The mass was determined using ASTRA software.
Low Molecular Weight Crystallin from Lenses Enhances Light Scattering by Denaturing Protein—We measured the light scattering ability of low molecular weight fractions (1–10-kDa fractions) isolated from young, adult, aged, and cataract human lenses using an ADH aggregation assay. As shown in the Table 1, the crystallin fragments present in 1–10-kDa fractions from young lenses showed the lowest light scattering activity compared with aged and cataract lens fractions. The cataract and aged lenses (≥70 years) showed 4.4–4.6-fold greater light scattering activity in them compared with young (14- and 16-year) lenses.
TABLE 1.
Effect of whole lens low molecular weight fraction on protein aggregation
The relative light scattering activity of low molecular weight fractions was estimated by the ADH aggregation assay described earlier (32). Low molecular weight crystallin fragments from the I–IV groups of lenses were concentrated to a final volume of 150 μl/lens, and 25-μl samples were used in assays. The result shown is an average from two assays. Group IV lenses are cataract lenses showing nuclear opacity with moderate yellow discoloration, similar to that observed in group III lenses.
| Group (age in years) | Increase in light scattering | Relative light scattering activity |
|---|---|---|
| % | ||
| I (14 and 16) | 5 | 1.0 |
| II (41 and 42) | 9 | 1.8 |
| III (70) | 23 | 4.6 |
| IV (70, 75, 79, and 89) | 22 | 4.4 |
Identification of Crystallin Peptides from Human Lenses—The low molecular weight peptide profiles of young, aged, and cataract lenses analyzed by MALDI-TOF MS are shown in Fig. 2, A–C, respectively. Within each lens group, the MALDI-TOF peptide profiles were comparable. The number of peptides in young lenses was less than that in aged lenses. Peptide signals were prominent in aged and cataract lenses but not in young lenses, suggesting that the total peptide concentration is higher in aged and cataract lenses than in young lenses. Peptide concentrations, determined in a single lens from each group, were 0.29, 0.9, and 1.1 mg/lens in young, aged, and cataract lenses, respectively. Peptides that were common to each group of lenses and smaller than 3500 Da were analyzed by nanoelectrospray ionization quadrupole time-of-flight tandem mass spectrometry to determine the sequence and origin of peptides. The peptides that were found in the young, aged, and aged cataract lenses and sequenced from MS/MS analysis are listed in Table 2. The GenBank™ accession numbers used to match the sequence of crystallin peptides are given in Table 2. The βA3/A1 peptide sequence was numbered based on a truncated βA3/A1 sequence submitted to GenBank™. The data indicate that crystallin fragments are generated from all major lens crystallins. Sequences could not be assigned to less abundant peptides identified by MALDI-TOF MS, and these peptides were excluded from the Table 2. Analysis of a 75-year-old lens showed that the concentration of LMW peptides is higher in the nucleus (931 μg) than in the outer cortex (338 μg). We also observed a differential distribution of individual peptides in the cortex and nucleus based on the MALDI-TOF MS signal intensity (data not shown). The signal intensity of αA-(43–56) and αB-(45–57) peptides was highest and quite prominent in the cortex. However, in the nucleus, these peptides had a weak signal (<4% of their signal intensity in the cortex). The peptides A, B, and αA-(66–80) were found only in the nucleus. Other peptides, including peptide G, were present in both regions, but their signal intensities were 5–10-fold higher in the nucleus.
FIGURE 2.
MALDI-TOF-MS of low molecular weight peptides isolated from young (32 years) (A), aged (94 years) (B), and cataract (87 years) (C) human lenses.
TABLE 2.
Low molecular weight peptides identified in the young, aged, and cataract human lenses using MALDI-TOF MS and quadrupole time-of-flight MS/MS
Peptides shown in boldface type were used in the current study. The peptide sequence was numbered based on the GenBank™ accession number given in the table.
|
Peptide mass
|
Source
|
Accession number
|
Found in
|
Peptide sequence from MS/MS analysis
|
||
|---|---|---|---|---|---|---|
| Young | Aged | Cataract | ||||
| 922.43 | αB-(59–66) | NP_001876 | No | Yes | Yes | PSWFDTGL |
| 1152.64 | αA-(67–75) | NP_000385 | Yes | No | No | DRDKFVIFL |
| 1239.67 | αA-(66–75) | NP_000385 | No | Yes | Yes | SDRDKFVIFL |
| 1381.64 | αB-(24–34) | NP_001876 | No | No | Yes | FDQFFGEHLLE |
| 1388.77 | γS-(167–178) | NP_060011 | Yes | Yes | Yes | SPAVQSFRRIVE |
| 1426.75 | αA-(2–12) | NP_000385 | No | Yes | Yes | DVTIQHPWFKR |
| 1527.81 | αA-(2–13) | NP_000385 | No | Yes | Yes | DVTIQHPWFKRT |
| 1550.84 | αB-(45–57) | NP_001876 | Yes | Yes | No | SPFYLRPPSFLRA |
| 1730.96 | αA-(43–56) | NP_000385 | Yes | Yes | Yes | TISPYYRQSLFRTV |
| 1778.91 | αA-(67–80) | NP_000385 | No | Yes | Yes | DRDKFVIFLDVKHF |
| 1835.01 | αA-(51–65) | NP_000385 | No | No | Yes | SrLFRTVLDSGISEVRa |
| 1847.99 | αA-(66–80) | NP_000385 | No | Yes | Yes | SDRDKFVIFLDVKHF-H20 |
| 1865.94 | αA-(66–80) | NP_000385 | No | Yes | Yes | SDRDKFVIFLDVKHF |
| 1938.43 | βA3/A1-(59–74) | AAC50971 | No | Yes | Yes | SD(N)AYHIERLMSFRPIC |
| 1963.07 | αA-(50–65) | NP_000385 | No | No | Yes | QSrLFRTVLDSGISEVRa |
| 2064.09 | βB2-(181–196) | AAB25691 | Yes | No | Yes | PQVQSVRRIRDMQWHQ |
| 2187.16 | αB-(2–18) | NP_001876 | No | Yes | Yes | DIAIHHPWIRRPFFPFH |
| 2359.18 | αB-(1–18) | NP_001876 | No | Yes | Yes | Ace-MDIAIHHPWIRRPFFPFH |
| 2598.35 | β(γ)S-(2–22) | P22914 | No | Yes | Yes | Ace-SKTGTKITFYEDKNFQGRRYD |
| 2930.62 | βB2-(181–204) | AAB25691 | Yes | No | Yes | PQVQSVRRIRDMQWHQRGAFHPSN |
| 3196.99 | βA3-(190–215) | NP_005199 | Yes | Yes | Yes | GDYKHWREWGSHAQTSQIQSIRRIQQ |
| 3252.65 | βA3-(189–215) | NP_005199 | Yes | Yes | Yes | GGDYKHWREWGSHAQTSQIQSIRRIQQ |
| 3389.74 | βA3-(188–215) | NP_005199 | Yes | Yes | Yes | HGGDYKHWREWGSHAQTSQIQSIRRIQQ |
MS/MS reported an extra Arg residue, which is shown as a lowercase letter
In vitro oxidized crystallin peptides were shown in our previous studies to interact with lens crystallins and to influence their aggregation and light scattering (27–29). The present study investigated whether in vivo found crystallin fragments interact with lens crystallins and produce similar effects on aggregation and light scattering. We selected the following three peptides for further study: αB-crystallin peptide (1MDIAIHHPWIRRPFFPFH18), βA3/A1-crystallin peptide (59SD(N)AYHIERLMSFRPIC74), and γS-crystallin peptide (167SPAVQSFRRIVE178), named peptide A, peptide B, and peptide G, respectively, based on the crystallin protein from which they were derived. A deamidated form of βA3/A1-(59–74) was used as peptide B. Peptides A and B are found only in the aged and cataract lenses, and peptide G is present in young, aged, and cataract lenses.
Crystallin Peptides Can Alter the Oligomerization of αB- and αA-crystallins—Incubation with peptide A or peptide B increased the mass distribution of αB-crystallin (Fig. 3, A and B). However, incubation with peptide G had no effect on the oligomeric mass of αB-crystallin (data not shown). Peptide A and peptide B increased the average oligomeric mass of αB-crystallin by 1.23- and 5.64-fold, respectively (Table 3). During incubation with peptide B, slight precipitation of αB-crystallin occurred. Elimination of the precipitate by centrifugation caused a decrease in the peak intensity of αB-crystallin-peptide B complex (Fig. 3B). The polydispersity of αB-crystallin increased in response to interaction with peptide A or B (Table 3). The hydrodynamic radius of αB-crystallin nearly doubled upon interacting with peptide B. αA-crystallin also exhibited an increase in the oligomeric mass but to a lesser extent upon interaction with peptide A or peptide B (data not shown). Unlike with αB-crystallin, αA-crystallin did not aggregate on interaction with peptide B. Analysis of the αA-peptide complex recovered during gel filtration studies by SDS-PAGE confirmed that only peptides A and B bind to αA-crystallin (Fig. 4). The results show that peptides A and B interact with αA- and αB-crystallin and alter their structure and dynamics, whereas peptide G has no effect.
FIGURE 3.
Increased oligomerization of αB-crystallin in response to interaction with peptides A and B. A, mass distribution across αB-crystallin peak with or without peptide A. B, mass distribution across αB-crystallin peak with or without peptide B. Chromatographic and analysis conditions are described in the legend to Fig. 1.
TABLE 3.
Effect of crystallin peptide interactions on the oligomeric size of αB-crystallin determined by multiangle light scattering
| Sample | Mass distribution across peak | Mass average | Polydispersity | Hydrodynamic radius |
|---|---|---|---|---|
| g/mol | g/mol | nm | ||
| αB-crystallin | 7.04e+5–5.58e+5 | 5.72e+5 (0.1%) | 1.003 (0.9%) | 8.1 (3%) |
| αB-crystallin + peptide B | 71.3e+5–29.8e+5 | 32.3e+5 (0.1%) | 1.056 (2%) | 15.9 (0.4%) |
| αB-crystallin + peptide A | 9.45e+5–6.14e+5 | 7.03e+5 (0.1%) | 1.006 (2%) | 8.3 (0.5%) |
FIGURE 4.
Demonstration of interaction of crystallin peptides A, B, and G with αA-crystallin. Incubated mixtures of αA-crystallin and each peptide were passed through a TSK G5000PWXL size exclusion column to separate the complex. The complex peak was analyzed using 4–20% Tris-HCl SDS-polyacrylamide gel electrophoresis. Lane 1, molecular mass markers; lane 2, αA-crystallin + peptide G; lane 3, αA-crystallin + peptide A; lane 4, αA-crystallin + peptide B. The arrow points at the peptide band.
Crystallin Peptides Enhance the Scattering of Light by Substrate Proteins—Studies of the effects of crystallin peptides on the thermal aggregation of substrate proteins demonstrated that βL- and γ-crystallins aggregated (scatter light) rapidly upon incubation at 55 °C. The addition of peptides to the incubation mixture increased the scattering of light by βL in a concentration-dependent manner (Fig. 5). At 60 μg of concentration, peptide A and peptide B augmented the scattering of βL-crystallin by 26 and 48%, respectively. However, peptide G at the same concentration caused only a marginal (6%) increase in the scattering by βL-crystallin. Crystallin peptides by themselves did not scatter light on incubation at 55 °C. The peptides had a similar effect on the thermal aggregation of γ-crystallin (data not shown). Light scattering and aggregation experiments were conducted for an extended time to determine the effect of peptides A, B, and C on βL-crystallin at physiological temperature. Tubes containing 250 μg of βL-crystallin were incubated with 100 μg of each peptide at 37 °C, and light scattering was monitored for up to 12 h. During 37 °C incubation, peptides A and B significantly enhanced βL-crystallin aggregation, whereas peptide G exerted very little influence (Fig. 6). The data suggest that βL- and γ-crystallin aggregation is enhanced upon interaction with peptide A or B. Incubation with peptide A and B at 37 °C also enhanced the scattering of light by ADH, a noncrystallin substrate protein generally used in aggregation studies (data not shown).
FIGURE 5.
Effects of increasing peptide concentrations on βL-crystallin aggregation at 55 °C. βL-Crystallin (100 μg) was incubated with various concentrations of peptide A, B, or G. A tube with βL alone served as control. Light scattering by βL-crystallin was monitored at 360 nm. The percentage of the increase in light scattering by βL-crystallin in presence of each peptide at 45 min was calculated and plotted.
FIGURE 6.
Facilitated aggregation of βL-crystallin in the presence of crystallin peptides A, B, and G at 37 °C. Peptide B had the greatest effect on aggregation. A, βL-crystallin alone (250 μg); B, βL-crystallin + 100 μg of peptide A; C, βL-crystallin + 100 μg of peptide B; D, βL-crystallin + 100 μg of peptide G; E, peptide A, B, or G (100 μg).
Enhanced precipitation of βL-crystallin as a result of peptide interaction was confirmed by analyzing the aggregation precipitate on reverse-phase HPLC on a C-18 column. Fig. 7 shows the protein elution profile of βL-crystallin precipitate before and after interaction with peptide B and peptide G. Protein elution profile was similar for βL-crystallin precipitate and peptide G-treated βL-crystallin. However, the HPLC profile showed increased amounts of protein in peptide B-treated βL-crystallin sample, suggesting enhanced aggregation. Although the concentration of every βL-crystallin subfraction increased in the peptide B-treated sample, the increase was significantly higher with βB2 crystallin. The data indicate that specific crystallin peptides found in aged lens can interact with intact crystallins, induce their aggregation, and lead to precipitation.
FIGURE 7.
Reverse-phase HPLC analysis of βL-crystallin aggregation precipitates in the presence of peptides A, B, and G. Black chromatogram, aggregation precipitate of βL-crystallin by itself; pink chromatogram, aggregation precipitate of βL-crystallin + peptide G; blue chromatogram, aggregation precipitate of βL-crystallin + peptide B.
Lens Crystallin Peptides Exhibit Anti-chaperone-like Activity—Anti-chaperone-like behavior of the crystallin fragments was demonstrated by performing the thermal aggregation assay in the presence of the peptide that modulated protein aggregation and oligomerization of chaperone protein α-crystallin (Fig. 8). Without peptide, α-crystallin suppressed the aggregation of βL-crystallin. However, the presence of peptide A in the aggregation system reduced α-crystallin chaperone activity. Similarly, peptide B showed anti-chaperone-like behavior with α-crystallin in a βL-crystallin aggregation assay. Suppression of αA-crystallin chaperone activity by peptide A was also confirmed by using ADH as the aggregating protein (Fig. 9). These results suggest that the accumulation of crystallin peptides in the lens may interfere with the chaperone property of α-crystallin.
FIGURE 8.
Inhibition of chaperone-like activity of α-crystallin against βL-crystallin by peptides A and B. Top, anti-chaperone activity of peptide A; bottom, anti-chaperone activity of peptide B. A, 100 μg of βL-crystallin; B, βL-crystallin + 25 μg of αA-crystallin; C, βL-crystallin + 60 μg of peptide; D, βL-crystallin + αA-crystallin + peptide; E, peptide alone (60 μg).
FIGURE 9.
Inhibition of chaperone-like activity of αB-crystallin by peptide B, measured against ADH substrate. A, ADH by itself, 250 μg; B, ADH + 20 μg of αB-crystallin; C, ADH + 20 μg of peptide B; D, ADH + αB-crystallin + 10 μg of peptide B; E, ADH + αB-crystallin + 20 μg of peptide B; F, peptide alone (20 μg).
Peptide Sequence Specificity in Protein Aggregation-enhancing Activity—Sequence specificity of peptide A and peptide B in influencing the aggregation of substrate proteins was determined by changing some of the residues in the native peptide sequence. We also synthesized peptides with the reverse sequence (retrosequence) of peptide A and peptide B and determined their aggregation-inducing activity. Table 4 lists the modified peptides that were tested for their influence on βL-crystallin aggregation. Replacement of hydrophobic residues with neutral or charged residues decreased or completely abolished the aggregation-inducing activity of peptide A and peptide B. Compared with native peptides, retropeptide A and retropeptide B showed 10 and 33% decrease in βL-crystallin aggregation-enhancing activity. The data show that variants of peptide A and peptide B sequences do not influence aggregation and light scattering to the same extent as corresponding native peptides, indicating the importance of the peptide sequence in crystallin aggregation. The data suggest that certain native lens peptides interact with lens crystallins, and such interactions may contribute to crystallin aggregation in vivo.
TABLE 4.
Comparison of the effect of native and mutant crystallin peptides on the scattering of light by βL-crystallin
βL-Crystallin (100 μg) was treated with 100 μg of native and mutant crystallin peptides and incubated at 53 °C. The scattering of light by βL was measured at 360 nm. The percentage increase in the light scattering against untreated βL was determined during a 45-min incubation period.
| Peptide | Sequence | Percentage increase in the light scattering by βL-crystallin |
|---|---|---|
| Native peptide A | MDIAIHHPWIRRPFFPFH | 73 |
| Mutant peptide A | MDIAGHHPWIRRPDFPAH | 3 |
| Mutant peptide A | MDIAIHHPAGRRPFFPFH | 58 |
| Retro peptide A | HFPFFPRRIWPHHIAIDM | 66 |
| Native peptide B | SD (N) AYHIERLMSFRPIC | 97 |
| Mutant peptide B | SD (N) ADHGERLMSFRPIC | 8 |
| Mutant peptide B | SD (N) ADHGERLMSFRPGC | 8 |
| Retro peptide B | CIPRFSMLREIHYA (N) DS | 65 |
DISCUSSION
Crystallin breakdown in the lens occurs for several reasons. Occasionally, it is the part of the normal lens maturation process (2). It may be due to the activation of lens proteolytic enzymes (17, 20, 33). Nonenzymatic mechanisms are also likely to generate crystallin fragments (34). Crystallin degradation is also necessary for the removal of denatured proteins or unfolded proteins that result from various oxidative stresses on lens. Proteasomes and ubiquitin systems in the lens are involved in the removal of oxidized proteins or peptides (35, 36). Despite this, truncated proteins/short peptides might accumulate in the lens because of their excessive production or because the degradation system cannot break down the fragments (37–40). Earlier studies showed that the lens contains significant quantities of crystallin fragments (6, 26), and the complex formed by the interaction of crystallin peptides with proteins in vivo can be modified further to form covalent multimers (5). Our study confirms that young, aged, and aged cataract lenses contain crystallin fragments of <3.5 kDa and that crystallin fragmentation increases with age. Further, the data included in our study (Table 1) show that both aged and cataract lenses have increased light scattering activity compared with the activity present in young lenses.
We could not relate the generation of LMW peptides to any specific protease. Of 23 LMW peptides that we identified, 15 peptides are derived from αA and αB-crystallin. Again, a majority of these peptides come from the N-terminal region, a few from the α-crystallin domain, and none from the C-terminal extension. Except for peptide B, all other peptides derived from β-or γ-crystallin are N- or C-terminal fragments. Interestingly, the subunit-interacting and substrate-interacting residue sequences identified by pin-array overlap with peptide A sequence (41, 42). The αB-(45–57) peptide found in the young and aged lenses forms part of the αA-crystallin recognition sequence in αB-crystallin (43). Peptides αA-(66–75) and αA-(66–80) have certain residues overlapping with the chaperone site sequence in αA-crystallin (44). Interactions between crystallins and peptides may be limiting the action of proteasome and peptidases on peptides, resulting in accumulation of peptides.
Crystallin peptides of aged lenses (peptide A and peptide B) increased the molecular mass, polydispersity, and hydrodynamic radius of αA- and αB-crystallins. The water-soluble fraction of aged lenses showed similar increases in protein molecular mass and polydispersity (Fig. 1). However, the mechanisms by which the peptides increase the molar mass of crystallin proteins in vivo are not known. This study also showed that binding of crystallin peptides decreased the ability of α-crystallin subunits to prevent aggregation of β- and γ-crystallins and other noncrystallin protein substrates, a process believed to be essential for lens transparency (Figs. 8 and 9). This study is the first to show that peptides generated in vivo can function as anti-chaperones. We previously demonstrated the anti-chaperone property of oxidized βL-crystallin peptides generated in vitro (27). Since peptide G did not interact with α-crystallin subunits, we believe that only certain crystallin fragments in the aged lens interact with intact α-crystallins and alter their structure and function.
To demonstrate the aggregation-enhancing property and anti-chaperone activity of the peptides within the short assay period, we have used a 2–10-fold molar excess of peptides over substrate protein concentration. We were unable to determine the exact concentration of individual peptides, but we observed that the crystallin fragments are not uniformly distributed in the lens. The concentration of peptides is higher in old lens fiber cells than in younger lens fiber cells (19). Compared with the clear region of the lens, the opaque region of the same lens possesses a higher concentration of crystallin fragments (45). MALDI tissue imaging of ocular lens also showed a differential distribution of truncated crystallins in the lens (46). Therefore, in isolated regions, the concentration of a peptide could reach to levels sufficient enough to produce the effect. Further, the presence of several peptides in lower concentration may be sufficient to induce protein aggregation due to cumulative effect of those peptides. Earlier study has shown that peptides on interaction induce a conformational change in the target protein that leads to an increased exposure of hydrophobic regions, resulting in an enhanced interaction and aggregation (29).
The process of aging and cataract development is a complex process involving multiple factors. This study suggests that accumulation of crystallin fragments is likely to be one of the causes of lens aging and opacification. The peptides generated in vivo can interact with β- and γ-crystallin and enhance their light scattering and precipitation. Interestingly, peptide B, which produced more effects than peptide A, is deamidated. Increasing concentrations of deamidated proteins and peptides have been demonstrated in the water-insoluble fractions of aged and cataract lenses (25, 47). To shorten the assay time in some of our experiments, we used temperatures above physiological levels to induce aggregation of β-or γ-crystallin and demonstrate the effects of interaction with peptides. Similar effects were produced at physiological temperature, however, but such assays required a longer duration. The mechanisms that underlie the release and accumulation of age-specific peptides in aged lenses are unknown. An explanation for their accumulation may be that, upon interaction with crystallins, peptides are not accessible to the peptidases that degrade them. The loss of peptidase activity in aged and cataract lenses could also lead to the accumulation of peptides (19, 40). The aggregation-influencing activity of peptides seems to be sequence-specific and lost almost completely when hydrophobic residues are replaced with neutral or charged residues. The order of the amino acids also seems to be a factor in aggregation, since the retro sequences of peptide A and peptide B showed diminished capacity to enhance βL-crystallin aggregation.
These studies provide the first evidence for the role of low molecular weight peptides in age-related cataract formation, one of the major protein aggregation diseases. The data suggest that a few polypeptides (crystallin fragments) generated in the lens during aging interact with crystallins and other proteins to increase their light scattering, facilitate their aggregation, and act as anti-chaperones. The results of this study of in vivo peptides support the hypothesis that crystallin fragments generated in vivo can facilitate protein aggregation. The initial process of cataract formation may involve peptide fragment-mediated loss of chaperone activity of α-crystallin, protein aggregation, and increased insolubility of lens crystallins. In previous studies, we showed that peptide interaction induces protein unfolding and exposure of hydrophobic sites (29). The exposed hydrophobic regions of the protein may interact with each other and further promote aggregation and increased insolubility. Taken together, our study suggests that an interaction of hydrophobic peptides with crystallins may be a key event in age-related protein aggregation, and cataract formation.
Acknowledgments
We thank Sharon Morey for help with preparation of the manuscript and Beverly DaGue (Proteomics Center, University of Missouri-Columbia) for performing mass spectrometry analysis.
This work is supported in part by National Institutes of Health Grants EY14795 and EY11981 and an unrestricted grant-in-aid from Research to Prevent Blindness (to the Department of Ophthalmology). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: ADH, alcohol dehydrogenase; MALDI-TOF, matrix-assisted laser desorption time-of-flight; MS, mass spectrometry; LMW, low molecular weight; HPLC, high pressure liquid chromatography.
References
- 1.Harding, J. J. (2002) Ageing Res. Rev. 1 465-479 [DOI] [PubMed] [Google Scholar]
- 2.Miesbauer, L. R., Zhou, X., Yang, Z., Yang, Z., Sun, Y., Smith, D. L., and Smith, J. B. (1994) J. Biol. Chem. 269 12494-12502 [PubMed] [Google Scholar]
- 3.Werten, P. J., Vos, E., and De Jong, W. W. (1999) Exp. Eye Res. 68 99-103 [DOI] [PubMed] [Google Scholar]
- 4.Hoehenwarter, W., Klose, J., and Jungblut, P. R. (2006) Amino Acids 30 369-389 [DOI] [PubMed] [Google Scholar]
- 5.Srivastava, O. P., Kirk, M. C., and Srivastava, K. (2004) J. Biol. Chem. 279 10901-10909 [DOI] [PubMed] [Google Scholar]
- 6.Srivastava, O. P. (1988) Exp. Eye Res. 47 525-543 [DOI] [PubMed] [Google Scholar]
- 7.Harrington, V., McCall, S., Huynh, S., Srivastava, K., and Srivastava, O. P. (2004) Mol. Vis. 10 476-489 [PubMed] [Google Scholar]
- 8.Lampi, K. J., Ma, Z., Hanson, S. R., Azuma, M., Shih, M., Shearer, T. R., Smith, D. L., Smith, J. B., and David, L. L. (1998) Exp. Eye Res. 67 31-43 [DOI] [PubMed] [Google Scholar]
- 9.Garber, A. T., Goring, D., and Gold, R. J. (1984) J. Biol. Chem. 259 10376-10379 [PubMed] [Google Scholar]
- 10.Ueda, Y., Duncan, M. K., and David, L. L. (2002) Invest. Ophthalmol. Vis. Sci. 43 205-215 [PubMed] [Google Scholar]
- 11.Thampi, P., Hassan, A., Smith, J. B., and Abraham, E. C. (2002) Invest. Ophthalmol. Vis. Sci. 43 3265-3272 [PubMed] [Google Scholar]
- 12.Shih, M., Lampi, K. J., Shearer, T. R., and David, L. L. (1998) Mol. Vis. 4 4. [PubMed] [Google Scholar]
- 13.David, L. L., Azuma, M., and Shearer, T. R. (1994) Invest. Ophthalmol. Vis. Sci. 35 785-793 [PubMed] [Google Scholar]
- 14.Zhan, H., Yamamoto, Y., Shumiya, S., Kunimatsu, M., Nishi, K., Ohkubo, I., and Kani, K. (2001) Histochem. J. 33 511-521 [DOI] [PubMed] [Google Scholar]
- 15.Swanson, A. A., Davis, R. M., and Meinhardt, N. C. (1985) Curr. Eye Res. 4 43-48 [DOI] [PubMed] [Google Scholar]
- 16.Mathur, P., Gupta, S. K., Wegener, A. R., Breipohl, W., Ahrend, M. H., Sharma, Y. D., Gupta, Y. K., and Vajpayee, R. B. (2000) Curr. Eye Res. 21 926-933 [DOI] [PubMed] [Google Scholar]
- 17.David, L. L., and Shearer, T. R. (1989) Lens Eye Toxic Res. 6 725-747 [PubMed] [Google Scholar]
- 18.Swaminathan, S., Chandrasekher, G., Venkataraman, A., and Pattabiraman, T. N. (1986) Biochem. Med. Metab. Biol. 35 184-190 [DOI] [PubMed] [Google Scholar]
- 19.Sharma, K. K., and Kester, K. (1996) Curr. Eye Res. 15 363-369 [DOI] [PubMed] [Google Scholar]
- 20.Wride, M. A., Geatrell, J., and Guggenheim, J. A. (2006) Birth Defects Res. C Embryo Today 78 90-105 [DOI] [PubMed] [Google Scholar]
- 21.Stadtman, E. R. (1990) Biochemistry 29 6323-6331 [DOI] [PubMed] [Google Scholar]
- 22.Friguet, B. (2002) Sci. World J. 2 248-254 [Google Scholar]
- 23.Grune, T., Reinheckel, T., Joshi, M., and Davies, K. J. (1995) J. Biol. Chem. 270 2344-2351 [DOI] [PubMed] [Google Scholar]
- 24.Roy, D., and Spector, A. (1978) Exp. Eye Res. 26 429-443 [DOI] [PubMed] [Google Scholar]
- 25.Hanson, S. R., Hasan, A., Smith, D. L., and Smith, J. B. (2000) Exp. Eye Res. 71 195-207 [DOI] [PubMed] [Google Scholar]
- 26.Srivastava, O. P., Srivastava, K., and Silney, C. (1996) Curr. Eye Res. 15 511-520 [DOI] [PubMed] [Google Scholar]
- 27.Senthilkumar, R., Chaerkady, R., and Sharma, K. K. (2002) J. Biol. Chem. 277 39136-39143 [DOI] [PubMed] [Google Scholar]
- 28.Udupa, E. G., and Sharma, K. K. (2005) Invest. Ophthalmol. Vis. Sci. 46 2514-2521 [DOI] [PubMed] [Google Scholar]
- 29.Udupa, P. E., and Sharma, K. K. (2005) Exp. Eye Res. 80 185-196 [DOI] [PubMed] [Google Scholar]
- 30.Das, K. P., and Surewicz, W. K. (1995) Biochem. J. 311 367-370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar, R. S., and Sharma, K. K. (2000) J. Pept. Res. 56 157-164 [DOI] [PubMed] [Google Scholar]
- 32.Santhoshkumar, P., and Sharma, K. K. (2006) Protein Sci. 15 2488-2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shih, M., David, L. L., Lampi, K. J., Ma, H., Fukiage, C., Azuma, M., and Shearer, T. R. (2001) Curr. Eye Res. 22 458-469 [DOI] [PubMed] [Google Scholar]
- 34.Voorter, C. E., de Haard-Hoekman, W. A., van den Oetelaar, P. J., Bloemendal, H., and de Jong, W. W. (1988) J. Biol. Chem. 263 19020-19023 [PubMed] [Google Scholar]
- 35.Shang, F., Nowell, T. R., Jr., and Taylor, A. (2001) Exp. Eye Res. 73 229-238 [DOI] [PubMed] [Google Scholar]
- 36.Huang, L. L., Shang, F., Nowell, T. R., Jr., and Taylor, A. (1995) Exp. Eye Res. 61 45-54 [DOI] [PubMed] [Google Scholar]
- 37.Hosler, M. R., Wang-Su, S. T., and Wagner, B. J. (2003) Int. J. Biochem. Cell Biol. 35 685-697 [DOI] [PubMed] [Google Scholar]
- 38.Ruotolo, R., Grassi, F., Percudani, R., Rivetti, C., Martorana, D., Maraini, G., and Ottonello, S. (2003) Mol. Vis. 9 538-548 [PubMed] [Google Scholar]
- 39.Zeng, J., Dunlop, R. A., Rodgers, K. J., and Davies, M. J. (2006) Biochem. J. 398 197-206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Viteri, G., Carrard, G., Birlouez-Aragon, I., Silva, E., and Friguet, B. (2004) Arch. Biochem. Biophys. 427 197-203 [DOI] [PubMed] [Google Scholar]
- 41.Ghosh, J. G., and Clark, J. I. (2005) Protein Sci. 14 684-695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghosh, J. G., Estrada, M. R., and Clark, J. I. (2005) Biochemistry 44 14854-14869 [DOI] [PubMed] [Google Scholar]
- 43.Sreelakshmi, Y., Santhoshkumar, P., Bhattacharyya, J., and Sharma, K. K. (2004) Biochemistry 43 15785-15795 [DOI] [PubMed] [Google Scholar]
- 44.Sharma, K. K., Kumar, R. S., Kumar, G. S., and Quinn, P. T. (2000) J. Biol. Chem. 275 3767-3771 [DOI] [PubMed] [Google Scholar]
- 45.Horwitz, J., Hansen, J. S., Cheung, C. C., Ding, L. L., Straatsma, B. R., Lightfoot, D. O., and Takemoto, L. J. (1983) Biochem. Biophys. Res. Commun. 113 65-71 [DOI] [PubMed] [Google Scholar]
- 46.Han, J., and Schey, K. L. (2006) Invest. Ophthalmol. Vis. Sci. 47 2990-2996 [DOI] [PubMed] [Google Scholar]
- 47.Wilmarth, P. A., Tanner, S., Dasari, S., Nagalla, S. R., Riviere, M. A., Bafna, V., Pevzner, P. A., and David, L. L. (2006) J. Proteome Res. 5 2554-2566 [DOI] [PMC free article] [PubMed] [Google Scholar]