Abstract
Although murine embryonic fibroblasts (MEFs) with Bax or Bak deleted displayed no defect in apoptosis signaling, MEFs with Bax and Bak double knock-out (DKO) showed dramatic resistance to diverse apoptotic stimuli, suggesting that Bax and Bak are redundant but essential regulators for apoptosis signaling. Chelerythrine has recently been identified as a Bcl-xL inhibitor that is capable of triggering apoptosis via direct action on mitochondria. Here we report that in contrast to classic apoptotic stimuli, chelerythrine is fully competent in inducing apoptosis in the DKO MEFs. Wild-type and DKO MEFs are equally sensitive to chelerythrine-induced morphological and biochemical changes associated with apoptosis phenotype. Interestingly, chelerythrine-mediated release of cytochrome c is rapid and precedes Bax translocation and integration. Although the BH3 peptide of Bim is totally inactive in releasing cytochrome c from isolated mitochondria of DKO MEFs, chelerythrine maintains its potency and efficacy in inducing direct release of cytochrome c from these mitochondria. Furthermore, chelerythrine-mediated mitochondrial swelling and loss in mitochondrial membrane potential (ΔΨm) are inhibited by cyclosporine A, suggesting that mitochondrial permeability transition pore is involved in chelerythrine-induced apoptosis. Although certain apoptotic stimuli have been shown to elicit cytotoxic effect in the DKO MEFs through alternate death mechanisms, chelerythrine does not appear to engage necrotic or autophagic death mechanism to trigger cell death in the DKO MEFs. These results, thus, argue for the existence of an alternative Bax/Bak-independent apoptotic mechanism that involves cyclosporine A-sensitive mitochondrial membrane permeability.
Mitochondria are the major organelles involved in the signal transduction and biochemical execution of apoptosis (1). Proteins of the Bcl-2 family are the central transducers of survival and apoptotic signals (2). They act at mitochondria by regulating the permeability and integrity of the mitochondrial outer membranes, thereby controlling the release of apoptogenic factors. The Bcl-2 family consists of three major subfamilies of pro-survival and pro-apoptotic molecules. Members of the BH3-only subfamily (Bim, Bad, Bid, Bik, Noxa, Puma, and Hrk) serve as sentinels for the initiation of apoptosis by modulating the function of members of the other two multidomain pro-survival (Bcl-2, Bcl-w, Mcl-1, Bcl-xL, and A1/Bfl-1) or pro-apoptotic (Bax and Bak) subfamilies (3-5).
A widely acknowledged paradigm of apoptotic signaling cascades suggests that apoptotic insults unleash one or more of the distinct BH3-only molecules and induce their translocation to the outer mitochondrial membrane (2, 6, 7). In mitochondria these proteins are thought to bind preferentially to the anti-apoptotic members of the Bcl-2 family (8-10) and, hence, serve to displace the multidomain pro-apoptotic members from binding to the pro-survival molecules. The ratio between the levels of multidomain pro-and anti-apoptotic members may, thus, play an important role in setting the rheostat for death responses to apoptotic insults (4, 11). Some of the BH3-only molecules such as Bid and certain splicing isoforms of Bim are thought to have an additional role in promoting activation of the multidomain pro-apoptotic molecules such as Bax and Bak (10, 12).
Bax and Bak are essential but redundant regulators of the mitochondrial apoptotic signaling pathway (8, 9, 13). Murine embryonic fibroblasts (MEFs)2 deficient in only one of the two molecules remain relatively normal in the execution of apoptosis signaling in response to a variety of apoptotic insults. Animals deficient in both molecules, however, display major abnormalities (14). Furthermore, MEFs derived from bax and bak double-knock-out mice (DKO) were found to be highly resistant to a wide variety of apoptotic stimuli, including overexpression of BH3-only molecules (8, 9, 13).
Despite the profound apoptotic defects, the ability of some bax and bak double-knock-out mice to survive into adulthood suggest the existence of other forms of cell death mechanism for directing proper development and maintaining tissue homeostasis by eliminating excess or damaged cells (14). Interestingly, several recent reports have shown that certain chemical apoptotic stimuli are capable of engaging in alternative forms of cell death in the absence of Bax and Bak. For example, DNA alkylating agents appeared to induce a mix of both apoptotic and necrotic cell death in MEFs, whereas they induced mainly necrotic death in MEFs lacking both Bax and Bak (15). Furthermore, although etoposide and staurosporine predominantly induce apoptosis in MEFs, they activate a caspase-independent cell death mechanism largely dependent on autophagy in the DKO MEFs (16). Therefore, the DKO MEFs could serve as a valuable tool in delineating multiple cell death mechanisms that can be engaged by cytotoxic agents which may otherwise escape detection in cells with intact apoptotic machinery.
Chelerythrine, a benzophenanthridine alkaloid, is known to trigger apoptosis in a variety of tumor cells (17). Recently, chelerythrine was identified by us as an inhibitory molecule that can block the heterodimerization of the Bcl-xL and Bak BH3 peptide in a high-throughput screen of 107,423 extracts derived from natural products (18). Although etoposide, staurosporine, and chelerythrine effectively triggered cytochrome c (cyt c) release from mitochondria in intact cells, only chelerythrine was able to induce cyt c release from isolated mitochondria (18), suggesting that chelerythrine acts directly on mitochondria. In this study we compared the cytotoxic activity of chelerythrine in the wild-type (WT) and DKO MEFs in an attempt to further delineate the mechanism by which chelerythrine mediates its cytotoxic effect in mammalian cells. Interestingly, chelerythrine is cytotoxic to both cell types. Surprisingly, the morphological and biochemical features associated with chelerythrine-induced cytotoxicity in DKO MEFs are indistinguishable from those observed in the WT MEFs. These are consistent with apoptotic rather than the autophagic or necrotic phenotypes associated with certain classic cytotoxic stimuli in the DKO MEFs. Furthermore, although chelerythrine and the Bim BH3 peptide are effective in triggering cyt c release from mitochondria isolated from WT MEFs, only chelerythrine remains active in mediating this effect on mitochondria isolated from the DKO MEFs. Chelerythrine induces mitochondrial swelling and loss of mitochondrial membrane potential (ΔΨm), and these effects are inhibited by cyclosporine A (CsA), suggesting the involvement of CsA-sensitive mitochondrial permeability transition pore (mPTP) components in both cell types. Taken together, these data strongly suggest that chelerythrine induces apoptosis in MEFs through a Bax- and Bak-independent mitochondrial mechanism that is distinct from the previously defined apoptosis gateway.
EXPERIMENTAL PROCEDURES
Reagents and Cell Lines—SV40 T antigen-transformed WT and DKO MEFs were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and antibiotics (100 μg of streptomycin/ml and 100 IU of penicillin/ml, Invitrogen). Lipofectamine (Invitrogen) was used for transfections according to the user's manual. The caspase inhibitors N-benzyloxycarbonyl-Val-Ala-Asp(O-Me) fluoromethyl ketone (zVAD-fmk) and N-(2-quinolyl)valyl-aspartyl-(2,6-difluorophenoxy)-methyl Ketone (Q-VD-OPH) were from Calbiochem. Staurosporine, etoposide, chelerythrine, camptothecin, 3-methyladenine (3MA), and CsA were from Sigma. Anti-cyt c antibody was purchased from BD Biosciences Pharmingen. Anti-Myc antibody was from Santa Cruz Biotechnology. Antibody against high mobility group B1 protein (HMG-B1) was from Abcam. Antibodies against actin and HSP60 were from Sigma.
Cyt c Release and Bax Translocation in Intact Cells—Cells were harvested and washed once with ice-cold phosphate-buffered saline (PBS). To separate the cytosolic fraction from other cellular components, cells were lysed for 5 min on ice in a solution consisting of 20 mm HEPES, pH 7.2, 50 mm KCl, 5 mm MgCl2, 1 mm EDTA, 1 mm EGTA, 250 mm sucrose, 200 μg/ml digitonin and protease inhibitors mixture as described (19). The organelles, cytoskeleton, and membranes were pelleted by centrifugation at 12,000 rpm for 10 min at 4 °C. The supernatant (cytosol) was carefully removed, and the pellet containing the mitochondria was solubilized in radioimmune precipitation assay buffer (150 mm NaCl, 0.1% SDS, 10% sodium deoxycholate, 1% Nonidet P-40, 2 mm EDTA, and 10 mm HEPES (pH 7.3)) containing the Complete protease inhibitors mixture. Protein content was determined by the Bradford reaction (Bio-Rad), and equivalent amount from each fraction were used for detection of cyt c release and Bax translocation using immunoblotting.
Analysis of Isolated Mitochondria—Mitochondria were isolated from MEFs as previously described (20). For in vitro cyt c release assays, cells were suspended in isolation buffer (320 mm sucrose, 1 mm EDTA, 50 mm HEPES (pH 7.5)) and disrupted by 20 expulsions through a 27-gauge needle. Disrupted cells were spun at 1000 × g for 10 min to remove cell debris and nucleus. The supernatants were centrifuged at 7000 × g for 10 min to pellet the heavy membrane fraction containing the mitochondria. The mitochondria-containing pellets were resuspended in assay buffer (250 mm sucrose, 2 mm KH2PO4, 5 mm sodium succinate, 25 mm EGTA, and 10 mm HEPES (pH 7.5)) at 0.5 mg/ml. Equal amounts of mitochondria were treated with the indicated compounds for 15 min at room temperature followed by centrifugation (20). Cyt c released into the supernatant was subjected to fractionation on 13.5% SDS-PAGE followed by Western blotting analysis. For light-scattering studies, mitochondrial isolated as described above were suspended in assay buffer (215 mm mannitol, 71 nm sucrose, 10 mm succinate, and 10 mm HEPES, pH 7.4) (21). Changes in absorbance at 540 nm (A540), indicating mitochondrial swelling as a consequence of mPTP opening, were measured after the addition of the indicated compounds using a microplate reader (Tecan). For dye retention assays, the mitochondrial suspension was incubated with 5,5′,6,6′-tetrachloro-1,1′,3,3′ tetraethylbenzimidazolyl-carbocyanine iodide (JC-1 100 ng/ml) for 10 min, then washed and resuspended in PBS; 5 min after the addition of indicated compounds, the level of JC-1 red fluorescence (FL2) retained by mitochondria was determined by flow cytometry (22).
Assessment of Cell Viability, Cell Proliferation, and Caspase 3/7 Activity—Cells were seeded onto 96-well plates. After 48 h they were treated with the indicated concentrations of compounds. Cell death was assessed using WST assay according to the supplier's protocols (Roche Applied Science). For the cell proliferation assay, cells were seeded onto 6-well dishes. After 2 h they were treated with the indicated compounds in the presence and absence of 3MA (10 mm). The cells were then recovered and re-cultured in standard medium onto 96-well plates. Viable cell numbers were measured on the indicated days by WST assay. Caspase 3/7 activity was measured using a Caspase-Glo 3/7 kit from Promega.
Reactive Oxygen Species (ROS) Measurement—Cells were treated with the indicated concentrations of compounds for 16 h. After such treatment, cells were washed in PBS and loaded with 1 μm 5-(and 6-)chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester dye (CM-H2DCFDA; Molecular Probes) in PBS for 30 min at 37 °C as described (23). The CM-H2DCFDA buffer was then removed, and the cells were washed twice with PBS before being analyzed by microplate reader (Tecan). Measurement was taken at an excitation wavelength of 500 nm and an emission wavelength of 520 nm. Emission from cells samples that were not loaded with CM-H2DCFDA was the same as for those with PBS.
Fluorescence Microscope Analysis—Nuclear DNA fragmentation was assessed by nuclear morphology after Hoechst 33342 staining. Cells were stained with 1 μm Hoechst 33342 for 5 min at room temperature. In another set of experiment designed for detecting early phase of apoptosis, cells were stained with annexin V-fluorescein isothiocyanate and propidium iodide by following the manufacturer's instructions (ApoAlert™ Annexin V Apoptosis kit, Clontech). Apoptotic cells were photographed using Zeiss epifluorescence microscope with an attached Nikon Coolpix digital camera.
Flow Cytometry—For the detection of sub-G1 DNA, cells were washed once, resuspended in 200 μl of PBS, and fixed in a 50-fold excess of ice-cold 70% ethanol. Cells were recovered by centrifugation at 1000 × g for 5 min at 4 °C, washed, stained with 50 mg/ml of propidium iodide for 30 min at room temperature, and analyzed in a FACScan flow cytometer (BD Biosciences). Mitochondrial membrane potential change (ΔΨm) as measured by JC-1 and tetramethylrhodamine ethyl ester staining was performed in accordance with the manufacturer's instructions (Molecular Probes). The exclusion assay for nonviable cells was performed by staining with 7-amino-actinomycin D (7AAD) followed by flow cytometry (BD Pharmingen). A minimum of 10,000 cells per sample were analyzed. The results presented are representative of at least three experiments.
RESULTS
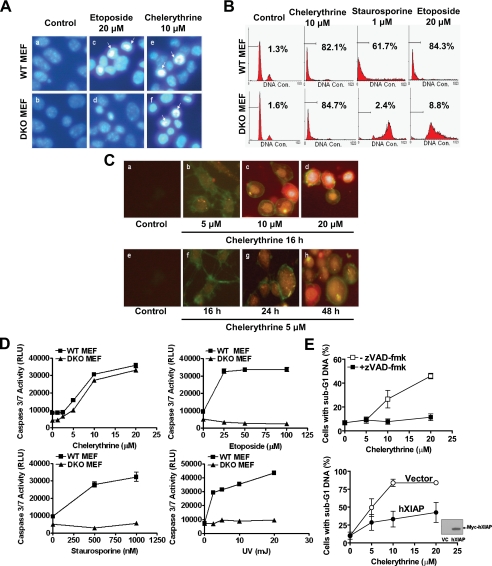
Chelerythrine Triggers Caspase-dependent Cytotoxicity in the Absence of Bax and Bak—In agreement with the published literature, DKO MEFs were found to be resistant to a variety of apoptotic stimuli including etoposide, staurosporine, camptothecin, cisplatin, methotrexate, and UV irradiation (Fig. 1, A, panel c and d, and B; data not shown). Surprisingly, in response to treatment with chelerythrine, both WT and DKO MEFs underwent similar morphological and biochemical changes that are hallmarks of apoptosis. The chelerythrine-treated MEFs displayed nuclear condensation (Fig. 1A, panels e and f) and the appearance of sub-G1 DNA (Fig. 1B). The appearance of sub-G1 DNA was seen in DKO MEFs treated with chelerythrine, but not etoposide, staurosporine, or camptothecin even though these compounds were fully effective in inducing sub-G1 DNA appearance in the WT MEFs (Fig. 1B; data not shown). Progressive stages of apoptosis could easily be detected in the chelerythrine-treated MEFs. At 5 μm, chelerythrine induced the appearance of annexin V-positive cells without an increase in propidium iodine staining at 16 h (Fig. 1C, panel b), indicative of early stage apoptosis. Cells exposed to 5 μm chelerythrine for prolonged period (Fig. 1C, panels g and h) or treated with higher concentrations of chelerythrine (Fig. 1C, panel c and d) became both annexin V- and propidium iodide-positive, presumably because of an increase in permeability of plasma membrane during the late stage of apoptosis. Moreover, chelerythrine was able to induce comparable caspase 3/7 activation in both WT and DKO MEFs (Fig. 1D). In contrast, etoposide, staurosporine, and UV irradiation were able to activate caspases 3/7 only in the WT MEFs (Fig. 1D). The appearance of sub-G1 DNA in DKO MEFs upon chelerythrine treatment was clearly caspase-dependent, as the effect was abolished by pretreatment with caspase inhibitor (zVAD-fmk) (Fig. 1E, upper panel) or overexpression of hXIAP (human X-linked inhibitor of apoptosis protein) (Fig. 1E, lower panel), a protein that is known to sequester and inhibit activated caspases (24). Chelerythrine failed to induce caspase-8 activation in both WT and DKO MEFs (data not shown), suggesting that the cytotoxic effect of chelerythrine in MEFs is not attributable to the extrinsic apoptosis signaling pathway.
FIGURE 1.
Chelerythrine-induced apoptosis is unaffected by the absence of Bax and Bak. A, chelerythrine induced nuclear condensation in DKO MEFs. MEFs were treated with 20 μm etoposide and 10 μm chelerythrine for 16 h. Nuclei were stained with Hoechst 33342 and then visualized using fluorescence microscopy. Arrows indicate condensed apoptotic nuclei. B, chelerythrine, but not staurosporine or etoposide, was effective in inducing accumulation of cells with sub-G1 DNA in DKO MEFs. MEFs were treated with etoposide (20 μm), staurosporine (1 μm), or chelerythrine (10 μm) for 48 h before they were ethanol-fixed and stained with propidium iodide for DNA and analyzed by flow cytometry. Percentages of cells with sub-G1 DNA are shown. Data are representative of at least three experiments. DNA Con., DNA content. C, chelerythrine was capable of inducing apoptotic phenotype on DKO MEFs. DKO MEFs were treated with the indicated concentrations of chelerythrine for 16 h or with 5 μm chelerythrine for the indicated durations. Cells were then incubated in binding buffer containing 0.2 μg/ml annexin V-fluorescein isothiocyanate and 10 μg/ml propidium iodide for 10 min and examined using fluorescence microscopy. Panel b and f show typical early apoptotic staining with green annexin V-fluorescein isothiocyanate, whereas the yellow signal in panels d and h show staining of the nuclei at late stages of apoptosis by which time the plasma membrane becomes permeable to propidium iodide. D, chelerythrine remains effective in inducing caspase 3/7 activation in the absence of Bax and Bak. MEFs were incubated with the indicated concentrations of chelerythrine, etoposide, staurosporine, or treated with indicated doses of UV irradiation. After 16 h, caspase 3/7 activities were measured according to caspase-Glo 3/7 kit (Promega). RLU, relative luminescence units. E, chelerythrine-induced accumulation of cells with sub-G1 DNA in DKO MEFs was efficiently blocked by zVAD-fmk (upper panel) and hXIAP overexpression (lower panel). DKO MEFs were treated with the indicated concentrations of chelerythrine in the presence (closed squares) or absence (open squares) of the broad spectrum caspase inhibitor zVAD-fmk (10 μm) or in the presence (closed circles) or absence (open circles) of hXIAP overexpression for 48 h. Cell lysates were fractionated by SDS-PAGE followed by Western blotting analyses using anti-Myc antibody to monitor the overexpression of Myc-tagged hXIAP (hXIAP). VC, vector control. DNA fragmentation was assessed using flow cytometry after ethanol-fixing and propidium iodide-staining. Percentages of cells with sub-G1 DNA (mean ± S.D., n = 3) are shown.
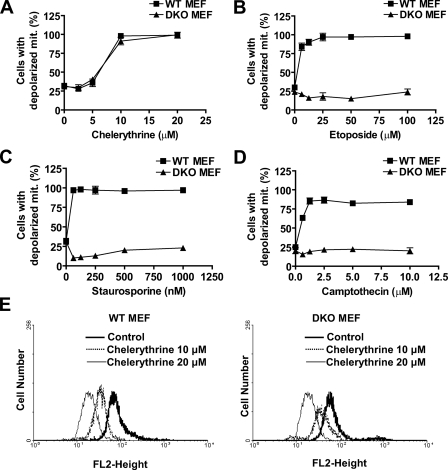
Chelerythrine Remains Fully Active in Inducing Loss of Mitochondrial Membrane Potential and Cyt c Release in the Absence of Bax and Bak—Bax and Bak are thought to be the gateway to regulate the release of apoptogenic factors from mitochondria. Because chelerythrine appears to induce apoptosis in cells that are deficient in these two proteins, we next evaluated mitochondrial apoptosis signaling in response to chelerythrine with the fluorescent dye JC-1 that allows the detection of loss of ΔΨm (mitochondrial depolarization). Treatment of WT and DKO MEFs with chelerythrine induced a substantial decrease in ΔΨm (Fig. 2A; supplemental Fig S1A, left panel). In contrast, etoposide (Fig. 2B; supplemental Fig S1A, right panel), staurosporine (Fig. 2C), camptothecin (Fig. 2D), and UV irradiation (data not shown) were ineffective in inducing loss of ΔΨm in the absence of Bax and Bak. The loss of ΔΨm induced by chelerythrine (Fig. 2E) but not by etoposide (supplemental Fig. S1B) in DKO MEFs was further confirmed using another fluorescent cationic dye, tetramethylrhodamine ethyl ester.
FIGURE 2.
Chelerythrine remains effective in inducing loss of ΔΨm in the absence of Bax and Bak. A-D, MEFs were treated with indicated concentrations of chelerythrine (A), etoposide (B), staurosporine (C), and camptothecin (D). After 16 h, cells were harvested, stained with JC-1, and analyzed by flow cytometry. The increases in JC-1 green fluorescence indicate the depolarized mitochondria. Percentages of cells with green fluorescence (Cells with depolarized mit.) upon treatment of WT or DKO MEFs with chelerythrine, etoposide, staurosporine, or camptothecin are plotted (mean ± S.D., n = 3). E, WT(left panel) and DKO (right panel) MEFs were treated with the indicated concentrations of chelerythrine. After 16 h cells were harvested, stained with 5 μm tetramethylrhodamine ethyl ester, and analyzed by flow cytometry. Fluorescence emissions (FL2-Height) of control (thick line), 10 μm (dotted line), and 20 μm (thin line) chelerythrine are shown. Chelerythrine-induced mitochondrial depolarization was indicated by reduced fluorescence intensity in the FL2 channel. Data are representative of at least three experiments.
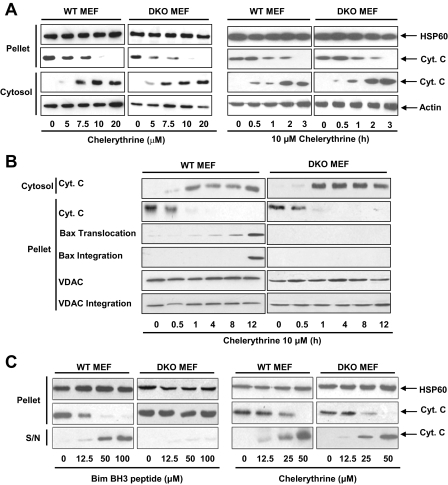
Consistent with the notion that the efficacy of chelerythrine in engaging mitochondrial apoptosis signaling is not affected by the absence of Bax and Bak, the kinetics and efficacy of cyt c release from mitochondria to cytosol triggered by chelerythrine in both WT and DKO MEFs were similar (Fig. 3A). Interestingly, chelerythrine appeared to cause rapid release of cyt c (Fig. 3A, right panel). Because translocation of Bax from cytosol to mitochondria and subsequent integration into outer mitochondrial membrane are thought to be the key signaling events preceding cyt c release upon apoptosis induction (20), we next compared the kinetics of these two events upon treatment with chelerythrine in WT and DKO MEFs. Surprisingly, chelerythrine-mediated Bax translocation and integration in the WT MEFs occurred at much slower kinetics than the cyt c release (Fig. 3B, left panel). In the DKO MEFs, despite the absence of Bax and Bak, the rapid kinetics of cyt c release was unaltered in response to chelerythrine (Fig. 3B, right panel). In contrast, staurosporine-induced Bax activation in WT MEFs coincided with cyt c release (supplemental Fig. S2A). DKO MEFs were significantly resistant to staurosporine-induced cyt c release (supplemental Fig. S2B), confirming the critical role of the Bax/Bak gateway in mediating staurosporine-induced apoptosis.
FIGURE 3.
The ability of chelerythrine in triggering cyt c release from intact cells and isolated mitochondria is not affected by the absence of Bax and Bak. A, Bax and Bak were dispensable for chelerythrine-induced cyt c release in intact cells. MEFs were treated with chelerythrine at the indicated concentrations for 16 h (left panel) or 10 μm chelerythrine for the indicated duration (right panel) and harvested for cytosolic and pellet fractions as described under “Experimental Procedures.” Proteins in the preparations were subjected to SDS-PAGE and immunoblotted with cyt c, actin (loading control for cytosolic fraction), and HSP60 antibodies (loading control for pellet fraction). B, chelerythrine induced rapid release of cyt c that preceded Bax translocation and integration in WT MEFs. MEFs were treated with 10 μm chelerythrine for the indicated durations and harvested for cytosolic and pellet fractions as described in A. For Bax and voltage-dependent anion channel (VDAC) integration analysis, mitochondria isolated from chelerythrine-treated (10 μm) WT and DKO MEFs for the indicated durations were resuspended in 0.1 m NaCO3, pH 10.5, and incubated on ice for 20 min followed by sonication for 5 min. Mitochondria were repelleted by centrifugation (100,000 rpm, 20 min) and immunoblotted for the indicated proteins. C, chelerythrine, but not Bim BH3 peptides, was efficacious in inducing cyt c release from mitochondria isolated from DKO MEFs. Mitochondria were isolated as described under “Experimental Procedures” and incubated at room temperature with the indicated concentrations of either Bim BH3 peptide (left panel) or chelerythrine (right panel). Supernatant (S/N), and pellet fractions containing mitochondria were then subjected to SDS-PAGE and immunoblotted with cyt c and HSP60 antibodies (loading control for pellet fractions). Data are representative of at least three experiments.
BH3-only molecules are thought to integrate and relay apoptosis signals to mitochondria. Polypeptides consisting of amino acids representing the sequence of the BH3 domains are sufficient to trigger the direct release of cyt c from isolated mitochondria (10). Certain chemical inhibitors of pro-survival members of Bcl-2 family including chelerythrine are thought to act as mimetic of the BH3-only molecules (18, 22, 25-30). Chelerythrine was indeed shown to be competent in releasing cyt c from isolated mitochondria (18). Consistent with the observation that BH3-only molecules are inactive in triggering apoptosis in DKO MEFs, the BH3 peptide encompassing the BH3 domain of Bim was effective only in releasing cyt c from mitochondria isolated from WT but not DKO cells (Fig. 3C, left panel). On the contrary, chelerythrine triggered the release of cyt c from mitochondria isolated from both WT and DKO MEFs with similar efficacy (Fig. 3C, right panel), suggesting that chelerythrine may act through a mechanism distinct from that of the BH3-only molecules.
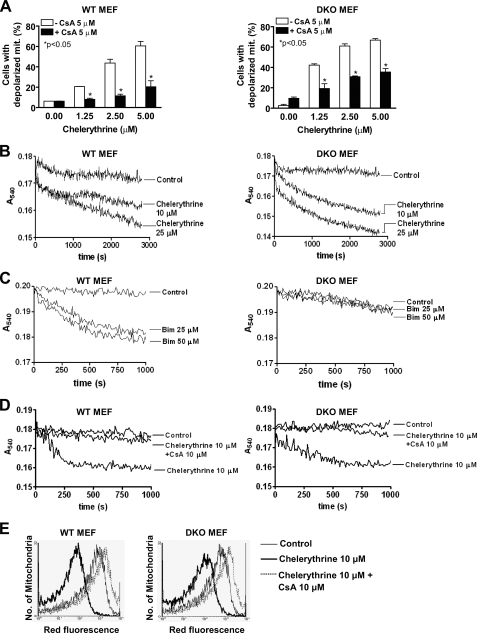
Chelerythrine Induces Cyclosporine A-sensitive Mitochondrial Permeability Transition Independent of Bax and Bak—Two major mechanisms have been proposed to account for the release of cyt c from mitochondria. One involves activation and oligomerization of Bax and Bak followed by direct pore formation by the oligomers (11, 31). Another proposed mechanism is the interaction of multidomain pro-apoptotic Bcl-2 family members with components of the mPTP, such as the voltage-dependent anion channel, the adenine nucleotide translocator, and cyclophilin D (CypD), which results in mitochondrial depolarization and swelling, followed by mitochondrial outer membrane rupture and release of the inter-membrane content (32). Because chelerythrine-mediated release of cyt c does not appear to be dependent on Bax and Bak, its effect on cyt c release may, therefore, involve certain components described in the latter mechanism. We next checked whether the ability of chelerythrine to induce loss in ΔΨm in MEFs is affected by the mPTP inhibitor CsA, which can act as a pseudo-substrate of CypD that prevents it from interacting with the mPTP (32). Pretreatment of WT (Fig. 4A, left panel) and DKO MEFs (Fig. 4A, right panel) with CsA was able to inhibit the increase in loss of ΔΨm induced by chelerythrine, suggesting chelerythrine may affect the mitochondrial integrity at least in part via the disruption of mPTP components.
FIGURE 4.
Chelerythrine directly induces CsA-sensitive mitochondrial permeability transition pore opening in the absence of Bax and Bak. A, chelerythrine-induced mitochondrial depolarization was inhibited by CsA in MEFs. WT (left panel) and DKO (right panel) MEFs were treated with the indicated concentrations of chelerythrine in the presence (closed bar) or absence (open bar) of CsA (5 μm) for 16 h. Cells were then harvested, stained with JC-1, and analyzed by flow cytometry. Data are shown as the mean ± S.D. (n = 3). Statistical analysis was performed by the Student's t test. B, chelerythrine induced mitochondrial swelling in WT and DKO MEFs. Mitochondria from WT (left panel) and DKO MEFs (right panel) were treated with the indicated concentrations of chelerythrine (10 and 25 μm) and monitored for mitochondrial swelling (by light scatter). C, Bim BH3 peptide induced mitochondrial swelling in WT but not DKO MEFs. Mitochondria from WT (left panel) and DKO MEFs (right panel) were treated with the indicated concentrations of Bim BH3 peptide (25 and 50 μm) and monitored for mitochondrial swelling (by light scatter). D, chelerythrine-induced swelling of mitochondria from WT and DKO MEFs was sensitive to CsA. Mitochondria isolated from WT (left panel) and DKO MEFs (right panel) were incubated for 15 min with CsA (10 μm) before the addition of 10 μm chelerythrine. Data from control non-treated mitochondria are also shown. Lines represent the values of one experiment performed at least three times. E, chelerythrine-induced CsA-sensitive loss of ΔΨm in mitochondria isolated from WT and DKO MEFs. Dye retention assays were carried out as described under “Experimental Procedures.” Histograms shown are control (thin lines) and 10 μm chelerythrine-treated mitochondria in the absence (thick line) or presence (dotted lines) of 10 μm CsA. Data show a decrease in red fluorescence indicating loss of ΔΨm in chelerythrine-treated in contrast to control mitochondria from WT or DKO MEFs, and the decrease was inhibited by CsA. Data are representative of at least three experiments.
To investigate further the role of mPTP in mediating the effect of chelerythrine, we used isolated mitochondria from WT and DKO MEFs to study its effect in causing mitochondrial swelling, which is indicative of the opening of mPTP. Direct addition of chelerythrine to mitochondria isolated from WT (Fig. 4B, left panel) and DKO (Fig. 4B, right panel) MEFs caused a fall in light attenuation in a dose-dependent manner, characteristic of large-amplitude swelling. In contrast, classic apoptotic stimuli (etoposide and staurosporine) failed to induce swelling of mitochondria isolated from both WT and DKO MEFs (data not shown). Bim BH3 peptide, on the other hand, was effective in inducing swelling of mitochondria isolated from WT MEFs (Fig. 4C, left panel) but not DKO MEFs (Fig. 4C, right panel), which supports the recent observation that Bim BH3 peptides can directly regulate Bax-mediated mitochondrial membrane permeabilization (33). Because chelerythrine-induced loss of ΔΨm was inhibited by CsA in intact cells, we next examined whether mitochondrial swelling can also be blocked by CsA. CsA completely attenuated chelerythrine-induced swelling of mitochondria isolated from WT (Fig. 4D, left panel) and DKO MEFs (Fig. 4D, right panel), providing further evidence to argue that chelerythrine induces mPTP opening by affecting mPTP components.
We also tested the effect of chelerythrine on mitochondrial membrane potential in isolated mitochondria. Isolated mitochondria were loaded with the ΔΨm-sensitive JC-1 probe before treatment, and mitochondrial labeling was determined by flow cytometry. Similar to the data in intact cells, chelerythrine induced a loss of ΔΨm in both isolated mitochondria from WT (Fig. 4E, left panel) and DKO MEFs (Fig. 4E, right panel). Consistent with the mitochondrial swelling observations, pretreatment of the mitochondria with CsA resulted in inhibition of loss of ΔΨm induced by chelerythrine (Fig. 4E). Collectively, our data indicate that chelerythrine has a direct effect on the CsA-sensitive, but Bax- and Bak-independent mPTP.
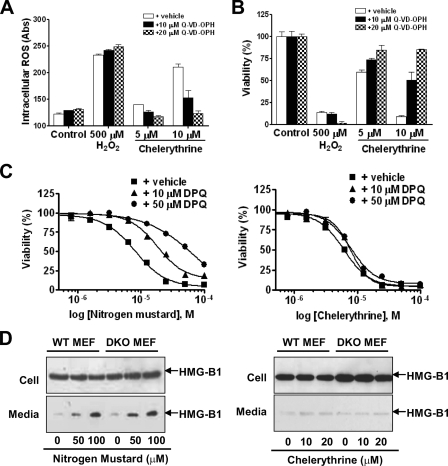
Chelerythrine Does Not Cause Necrotic Death—Although it has been well documented that the CsA-sensitive mPTP plays a role in mitochondrial apoptotic signaling, recent data from CypD-deficient mice showed that CypD-deficient mitochondria did not undergo CsA-sensitive mPTP and loss of ΔΨm. These CypD-deficient MEFs were resistant only to necrotic stimuli, suggesting that necrosis, but not apoptosis, is regulated by the CsA-sensitive mPTP (34). Because chelerythrine-induced loss of ΔΨm and mitochondrial swelling can be inhibited by CsA (Fig. 4), it raises the question of whether chelerythrine-mediated cytotoxicity may at least in part associate with necrotic mechanism. To examine necrosis as a contributing factor of chelerythrine-mediated cytotoxicity, we first compared the cytotoxicity of chelerythrine, and the necrotic death resulted from exposure to high concentration of hydrogen peroxides (500 μm H2O2) (34, 35). As expected, high levels of H2O2 failed to induce apoptosis in DKO MEFs as indicated by the absence of caspases activation, sub-G1 DNA appearance, and nuclear fragmentation (data not shown). H2O2, however, was capable of triggering ROS production (Fig. 5A; supplemental Fig. S3A) and cell death (Fig. 5B). Although chelerythrine was also capable of elevating ROS level, a broad spectrum caspase inhibitor (Q-VD-OPH) was effective in ablating chelerythrine-induced, but not H2O2-induced ROS elevation (Fig. 5A; supplemental Fig. S3A) and cell death (Fig. 5B), suggesting that mitochondria-derived ROS originated from caspase feedback mechanism may play a role in chelerythrine, but not H2O2-induced cell death in MEFs.
FIGURE 5.
Chelerythrine does not trigger necrosis in DKO MEFs. A-B, the broad spectrum caspase inhibitor, Q-VD-OPH, inhibited ROS elevation and cell death triggered by chelerythrine but not H2O2 in DKO MEFs. DKO MEFs were treated with H2O2 (500 μm) or chelerythrine (5 and 10 μm) in the presence (filled bar, 10 μm; cross-hatched bar, 20 μm) or absence (open bar) of Q-VD-OPH for 16 h. Cells were then subjected to measurement of intracellular ROS level using CM-H2DCFDA staining detected by microplate reader (A) and viability analysis using WST assay (B). C, PARP inhibition resulted in resistance to nitrogen mustard but not chelerythrine-induced cell death in DKO MEFs. DKO MEFs were treated with indicated concentrations of nitrogen mustard or chelerythrine in the presence (triangles, 10 μm; circles, 50 μm) or absence (squares) of PARP inhibitor (DPQ) for 4 h. Cell viability was then determined using WST assay. D, HMG-B1 was released into extracellular environment during nitrogen mustard- but not chelerythrine-induced cell death in MEFs. WT and DKO MEFs were treated with the indicated concentrations of nitrogen mustard (left panel) or chelerythrine (right panel) for 16 h. Culture media were collected after treatment, and cells were lysed in radioimmune precipitation assay buffer. HMG-B1 in both cell lysates and culture media was detected by immunoblotting after SDS-PAGE fractionation.
Interestingly, another regulated form of necrotic death that is independent of Bax/Bak mitochondrial apoptosis pathway but is dependent on poly(ADP-ribose) polymerase (PARP) activation has recently been described in cells treated with alkylating DNA damaging agents (15). In agreement with the reported data, PARP inhibitor (DPQ) was effective in partial rescue of cell death induced by alkylating DNA damage agent mechlorethamine hydrochloride (nitrogen mustard) in WT (supplemental Fig. S3B, left panel) and DKO MEFs (Fig. 5C, left panel). PARP inhibitor (DPQ, however, did not appear to have a significant effect on blocking chelerythrine-mediated cytotoxicity in either WT (supplemental Fig. S3B, right panel) or DKO MEFs (Fig. 5C, right panel). It has been reported that extracellular HMG-B1 is responsible for the inflammatory response of cell necrosis (36). Necrotic death induced by nitrogen mustard involves the release of HMG-B1 into the extracellular environment, which acts as a ligand for the monocyte/macrophage scavenger receptor RAGE (15). Therefore, cells that were treated with nitrogen mustard or chelerythrine were also evaluated for their effect on affecting the localization of HMG-B1. HMG-B1 was found in the extracellular environment of cells treated with nitrogen mustard (Fig. 5D, left panel). In contrast, chelerythrine failed to induce the release of HMG-B1 into the extracellular environment (Fig. 5D, right panel). Together, these data suggest that chelerythrine-mediated cell death does not involve the necrotic mechanism associated with caspase-independent reactive oxygen species or alkylating agent.
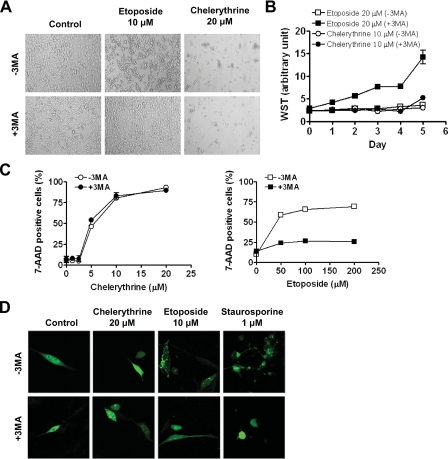
Chelerythrine-induced Cytotoxicity Is Not Attributable to Autophagy—It has recently been shown that cytotoxic drugs could have dual mechanisms in conferring cytotoxicity in mammalian cells. For example, when the apoptotic effect of etoposide is completely lost in the DKO MEFs, it remains cytotoxic to these cells (16). Electron microscopic and biochemical studies revealed that the etoposide-induced cell death in DKO MEFs was associated with autophagosomes/autolysosomes and was effectively suppressed by inhibitors of autophagy, including 3MA in these cells (16). Surprisingly, the autophagic death mediated by etoposide appears to be positively regulated by Bcl-2 and Bcl-xL (16). Because chelerythrine targets Bcl-xL (18, 37), autophagic mechanism can potentially be involved in chelerythrine-mediated cytotoxicity in the DKO MEFs. We, therefore, tested the ability of 3MA to inhibit chelerythrine-induced cell death. Similar to reported results, 3MA was effective in preventing the rounding up of DKO MEFs upon etoposide treatment (Fig. 6A). However, 3MA appeared to be totally ineffective in blocking the effect of chelerythrine on both the WT (data not shown) and DKO MEFs (Fig. 6A). The ability of chelerythrine- or etoposide-treated cells to proliferate was also investigated. 3MA treatment did not rescue the DKO MEFs treated with chelerythrine, whereas it significantly enhanced the proliferating capacity of etoposide-treated cells (Fig. 6B). Furthermore, 3MA also failed to improve the viability of chelerythrine-treated cells as determined using WST assay (data not shown). Cells undergoing autophagic death are known to have compromised plasma membrane integrity. The ability of DKO MEFs to exclude the DNA dye 7AAD upon chelerythrine or etoposide treatment in the presence or absence of 3MA was evaluated. Etoposide-treated DKO MEFs were able to exclude 7AAD in the presence of 3MA (Fig. 6C, right panel), whereas the plasma membrane of chelerythrine-treated WT (data not shown) and DKO MEFs (Fig. 6C, left panel) was still permeable to 7AAD with the 3MA treatment. During the autophagic process, microtubule light chain-3 (LC3) concentrated in autophagosomes, which would appear as punctuate rather than diffuse staining patterns under immunofluorescence microscopy (38). Diffuse cytoplasmic localization of transiently expressed LC3 was observed in healthy and chelerythrine-treated DKO MEFs, whereas etoposide- and staurosporine-treated DKO MEFs showed punctuate fluorescence staining of LC3 under immunofluorescence microscopy (Fig. 6D). Etoposide- and staurosporine-induced aggregation of LC3 was inhibited by 3MA. In contrast, 3MA had no effect on LC3 localization either in healthy or chelerythrine-treated DKO MEFs (Fig. 6D). In addition, 3MA was also unable to block chelerythrine-induced mitochondrial membrane potential change (supplemental Fig. S4). Together, these data reveal no evidence that chelerythrine can mediate autophagic death in either the WT or DKO MEFs.
FIGURE 6.
Autophagic mechanism does not contribute significantly to the cytotoxic effect of chelerythrine in DKO MEFs. A-C, 3MA blocked cell death triggered by etoposide but not chelerythrine in DKO MEFs. A, DKO MEFs were treated with 10 μm etoposide or 20 μm chelerythrine in the absence (-3MA) or presence (+3MA) of 10 mm 3MA for 48 h and then examined by phase-contrast microscopy. B and C, DKO MEFs were treated with indicated concentrations of chelerythrine (circle symbols) or etoposide (square symbols) in the absence (open symbols) or presence (closed symbols) of 3MA. Cell viability was measured by a cell proliferation assay after re-seeding the cells for the indicated days followed by WST assay (B) and 7AAD exclusion staining (C); cell death was expressed as percentage of 7AAD positive cells. Data shown are the mean ± S.D. (n = 3). D, chelerythrine did not induce punctuate distribution of LC3 in DKO MEFs. MEFs transiently transfected with pXJ-myc-LC3 were treated with chelerythrine (20 μm), etoposide (10 μm), or staurosporine (1 μm) for 16 h, and cells were fixed and permeabilized for immunofluorescence confocal microscopy using anti-Myc epitope antibody.
DISCUSSION
Chelerythrine was identified as Bcl-xL inhibitor from a high throughput screen and was subsequently shown that it can interact with the BH groove instead of the classic BH3 binding cleft when many other chemical inhibitors of Bcl-2/Bcl-xL are known to bind. Chelerythrine can act on isolated mitochondria from tumor cells for triggering the direct release of cyt c (18). In this study we characterized and compared the cytotoxic mechanism of chelerythrine in both WT and DKO MEFs. In contrast to other established chemical apoptotic stimuli, chelerythrine appears to induce classic apoptotic phenotypes in both cell types with similar kinetics and efficacy. Although chelerythrine was capable of inducing Bax integration in WT MEFs, this event occurred only after a long delay (∼10 h after cyt c release), suggesting that Bax activation in the WT MEFs may occur via positive feedback mechanism triggered by downstream apoptosis events. Furthermore, the kinetics of cyt c release from mitochondria isolated from both WT and DKO MEFs were almost identical, suggesting that Bax activation may not be a major contributing factor in mediating the apoptotic phenotypes triggered by chelerythrine. Indeed, the rapid release of cyt c that preceded Bax activation and the inhibitory activity of CsA toward mitochondrial swelling strongly suggest that chelerythrine-induced apoptosis in MEFs is predominantly due to the CsA-sensitive mPTP opening instead of the Bax/Bak-mediated permeabilization mechanism.
Because chelerythrine is able to interact with Bcl-xL and acts directly on mitochondria (18), it is tempting to speculate that the effect of chelerythrine on the mPTP is related to Bcl-2/Bcl-xL inhibition. Although the molecular mechanism by which Bcl-2/Bcl-xL inhibits mPTP is still controversial, several independent evidence have emerged demonstrating that these proteins can interact with sessile mitochondrial proteins, including adenine nucleotide translocator (39) and voltage-dependent anion channel (40). In vitro, overexpression of Bcl-2 in cells or the addition of Bcl-2 to isolated mitochondria suppressed the mPTP induced by a variety of apoptotic insults (39, 41, 42). Therefore, it is plausible that the inhibition of Bcl-2/Bcl-xL by chelerythrine might result in the sensitization of the mitochondrial membranes permeability via mPTP components, release of cyt c, and functional collapse of the organelle and apoptosis. However, alternative mechanisms such as activation of other unidentified mitochondrial targets that are dependent on mPTP components by chelerythrine are certainly possible and remain to be explored.
Although we were investigating the Bax- and Bak-independent apoptotic mechanism triggered by chelerythrine, two studies were published to suggest that two distinct apoptotic insults, such as gossypol and A23187/arachidonic acid, are capable of inducing apoptosis in the DKO MEFs. In contrast to chelerythrine, the apoptotic effects of gossypol (supplemental Fig. S5A) and the A23187/arachidonic acid (43) are significantly weaker in the DKO than the WT MEFs, suggesting that the apoptotic effects emulated from these stimuli may in part still be dependent on the Bax/Bak gateway. Furthermore, our data showed that neither gossypol (supplemental Fig. S5, B and C) nor A23187/arachidonic acid (43) was effective in triggering cyt c release and swelling in isolated mitochondrial preparations, suggesting that unlike chelerythrine, these compounds do not act directly on mitochondria to mediate their apoptotic effect in MEFs. In fact, our results are in disagreement with a previous report showing that gossypol was able to induce cyt c release from mitochondria isolated from DKO MEFs (44). In the study by Lei et al. (44), mitochondria were incubated for 1 h in a buffer devoid of calcium chelator EGTA, whereas our experiments were performed for 30 min in a buffer containing EGTA. This may explain why their mitochondrial preparation was more sensitive to gossypol, as it has been documented that mitochondria have a tendency to release spontaneously high levels of cyt c in the absence of EGTA (45). The mechanistic basis for the mitochondrial effects of these drugs also appears to be different as gossypol- and A23187/arachidonic acid-induced cyt c release are insensitive to CsA and independent of mPTP opening (43, 44), whereas the mitochondrial event induced by chelerythrine is mediated by CsA-sensitive mPTP.
Recently, the ability of several chemical inhibitors of Bcl-2/Bcl-xL (ABT-737, BH3I-1, HA14-1, antimycin A, gossypol, and chelerythrine) to induce cell death in the DKO MEFs has also been investigated (46). Except ABT-737, these inhibitors appeared cytotoxic to DKO MEFs. However, the nature of the cytotoxic mechanisms associated with each of these inhibitors in DKO MEFs was not known as detailed phenotypic, and molecular characterizations were not performed. It was concluded from that study that only ABT-737 qualifies as a genuine chemical BH3 mimetic as it behaves just like BH3-only proteins in that they fail to induce apoptosis in the absence of Bax and Bak. Surprisingly, our in-depth characterization and comparison of cytotoxic mechanism of chelerythrine in WT and DKO MEFs provides strong and comprehensive evidence to suggest that chelerythrine is an apoptotic agent that acts through Bax/Bak-independent mitochondrial mechanism. As revealed by NMR and modeling analysis, chelerythrine can induce a global change in the Bcl-xL protein structure through direct interaction with the BH groove (37) instead of the classic BH3 domain binding cleft where BH3 peptides and ABT-737 are shown to dock (26). This finding would suggest that chelerythrine may confer its inhibitory action on Bcl-xL by directly or indirectly competing with unknown regulatory proteins. It would be of interest to investigate further on the structure activity relationship of chelerythrine-related compounds on their apoptotic effect in the DKO MEFs versus their binding and inhibitory activities on pro-survival members of Bcl-2 family to gain further insight on whether the Bax/Bak-independent pro-apoptotic mechanism is dependent on direct interaction with the pro-survival molecules.
Chelerythrine has recently been shown to display a biphasic effect on mitochondrial respiration with energy uncoupling at low concentration and respiration inhibition at high concentration (47). Based on this study, chelerythrine-induced apoptosis in MEFs might just be a simple consequence of mitochondrial toxicity. Interestingly, in contrast to chelerythrine, respiratory complex IV inhibitor (sodium azide) (48) and uncoupler of mitochondrial oxidation (carbonyl cyanide p-trifluoromethoxyphenylazone) (49) that directly interfere energy coupling were cytotoxic to WT but not the DKO MEFs (supplemental Fig. S6). These data would serve to argue that the main mode of action of chelerythrine in causing cytotoxicity in MEFs is unlikely due to its effect on mitochondrial respiration.
It was suggested that Bax and Bak might share a redundant function that suppresses tumorigenicity through the independent ability of either of these proteins to initiate apoptosis in response to oncogenic transformation (9). Using transformed primary baby kidney epithelial cells, Degenhardt et al. (50) also showed that Bax and Bak function to suppress tumorigenesis, and their deficiency resulting from gene mutation was found to be prevalent in tumor cells detected in metastatic sites in a mouse tumor model. Therefore, designing specific strategies to overcome survival of the tumor cells in the absence of Bax and Bak function may provide novel opportunities in anticancer drug discovery. Indeed, it has been shown that chelerythrine induces tumor growth delays in vivo and appears to have a minimum adverse effect in mice (51). In this respect chelerythrine may have the potential to serve as a lead compound to support exploration of the Bax/Bak-independent mechanism as a molecular target for anticancer drug development.
Supplementary Material
Acknowledgments
We are grateful to the late Dr. Stanley Korsmeyer for providing the DKO MEFs. We thank NaiYang Fu for valuable comments on the manuscript.
This work was supported by the Biomedical Research Council of A*STAR (Agency for Science, Technology, and Research), Singapore. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1-S6.
Footnotes
The abbreviations used are: MEF, murine embryonic fibroblast; WT, wild type; DKO, Bax and Bak double knockout; cyt c, cytochrome c; ΔΨm, mitochondrial membrane potential; CsA, cyclosporine A; mPTP, mitochondrial permeability transition pore; hXIAP, human X-linked inhibitor of apoptosis protein; CypD, cyclophilin D; PBS, phosphate-buffered saline; ROS, reactive oxygen species; PARP, poly(ADP-ribose) polymerase; Q-VD-OPH, N-(2-quinolyl)valyl-aspartyl-(2,6-difluorophenoxy) methyl ketone; zVAD-fmk, N-benzyloxycarbonyl-Val-Ala-Asp(O-Me) fluoromethyl ketone; 3MA, 3-methyl adenine; JC-1, 5,5′,6,6′-tetrachloro-1,1′,3,3′ tetraethyl-benzimidazolylcarbocyanine iodide; 7AAD, 7-amino-actinomycin D; CM-H2DCFDA, 5-(and 6-)chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester dye; HMG-B1, high mobility group B1 protein; LC3, light chain-3; WST, water-soluable tetrazolium salts.
References
- 1.Wang, X. (2001) Genes Dev. 15 2922-2933 [PubMed] [Google Scholar]
- 2.Chan, S. L., and Yu, V. C. (2004) Clin. Exp. Pharmacol. Physiol. 31 119-12815008953 [Google Scholar]
- 3.Strasser, A. (2005) Nat. Rev. Immunol. 5 189-200 [DOI] [PubMed] [Google Scholar]
- 4.Danial, N. N., and Korsmeyer, S. J. (2004) Cell 116 205-219 [DOI] [PubMed] [Google Scholar]
- 5.Willis, S. N., and Adams, J. M. (2005) Curr. Opin. Cell Biol. 17 617-625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouillet, P., and Strasser, A. (2002) J. Cell Sci. 115 1567-1574 [DOI] [PubMed] [Google Scholar]
- 7.Willis, S. N., Fletcher, J. I., Kaufmann, T., van Delft, M. F., Chen, L., Czabotar, P. E., Ierino, H., Lee, E. F., Fairlie, W. D., Bouillet, P., Strasser, A., Kluck, R. M., Adams, J. M., and Huang, D. C. (2007) Science 315 856-859 [DOI] [PubMed] [Google Scholar]
- 8.Cheng, E. H., Wei, M. C., Weiler, S., Flavell, R. A., Mak, T. W., Lindsten, T., and Korsmeyer, S. J. (2001) Mol. Cell 8 705-711 [DOI] [PubMed] [Google Scholar]
- 9.Zong, W. X., Lindsten, T., Ross, A. J., MacGregor, G. R., and Thompson, C. B. (2001) Genes Dev. 15 1481-1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Letai, A., Bassik, M. C., Walensky, L. D., Sorcinelli, M. D., Weiler, S., and Korsmeyer, S. J. (2002) Cancer Cell 2 183-192 [DOI] [PubMed] [Google Scholar]
- 11.Reed, J. C. (1998) Oncogene 17 3225-3236 [DOI] [PubMed] [Google Scholar]
- 12.Kim, H., Rafiuddin-Shah, M., Tu, H. C., Jeffers, J. R., Zambetti, G. P., Hsieh, J. J., and Cheng, E. H. (2006) Nat. Cell Biol. 8 1348-1358 [DOI] [PubMed] [Google Scholar]
- 13.Wei, M. C., Zong, W. X., Cheng, E. H., Lindsten, T., Panoutsakopoulou, V., Ross, A. J., Roth, K. A., MacGregor, G. R., Thompson, C. B., and Korsmeyer, S. J. (2001) Science 292 727-730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindsten, T., Ross, A. J., King, A., Zong, W. X., Rathmell, J. C., Shiels, H. A., Ulrich, E., Waymire, K. G., Mahar, P., Frauwirth, K., Chen, Y., Wei, M., Eng, V. M., Adelman, D. M., Simon, M. C., Ma, A., Golden, J. A., Evan, G., Korsmeyer, S. J., MacGregor, G. R., and Thompson, C. B. (2000) Mol. Cell 6 1389-1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zong, W. X., Ditsworth, D., Bauer, D. E., Wang, Z. Q., and Thompson, C. B. (2004) Genes Dev. 18 1272-1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimizu, S., Kanaseki, T., Mizushima, N., Mizuta, T., Arakawa-Kobayashi, S., Thompson, C. B., and Tsujimoto, Y. (2004) Nat. Cell Biol. 6 1221-1228 [DOI] [PubMed] [Google Scholar]
- 17.Malikova, J., Zdarilova, A., Hlobilkova, A., and Ulrichova, J. (2006) Cell Biol. Toxicol. 22 439-453 [DOI] [PubMed] [Google Scholar]
- 18.Chan, S. L., Lee, M. C., Tan, K. O., Yang, L. K., Lee, A. S., Flotow, H., Fu, N. Y., Butler, M. S., Soejarto, D. D., Buss, A. D., and Yu, V. C. (2003) J. Biol. Chem. 278 20453-20456 [DOI] [PubMed] [Google Scholar]
- 19.Wilson-Annan, J., O'Reilly, L. A., Crawford, S. A., Hausmann, G., Beaumont, J. G., Parma, L. P., Chen, L., Lackmann, M., Lithgow, T., Hinds, M. G., Day, C. L., Adams, J. M., and Huang, D. C. (2003) J. Cell Biol. 162 877-887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan, K. O., Fu, N. Y., Sukumaran, S. K., Chan, S. L., Kang, J. H., Poon, K. L., Chen, B. S., and Yu, V. C. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 14623-14628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galindo, M. F., Jordan, J., Gonzalez-Garcia, C., and Cena, V. (2003) Br. J. Pharmacol. 139 797-804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tzung, S. P., Kim, K. M., Basanez, G., Giedt, C. D., Simon, J., Zimmerberg, J., Zhang, K. Y., and Hockenbery, D. M. (2001) Nat. Cell Biol. 3 183-191 [DOI] [PubMed] [Google Scholar]
- 23.Kroll, S. L., and Czyzyk-Krzeska, M. F. (1998) Am. J. Physiol. 274 C167-C174 [DOI] [PubMed] [Google Scholar]
- 24.Srinivasula, S. M., Hegde, R., Saleh, A., Datta, P., Shiozaki, E., Chai, J., Lee, R. A., Robbins, P. D., Fernandes-Alnemri, T., Shi, Y., and Alnemri, E. S. (2001) Nature 410 112-116 [DOI] [PubMed] [Google Scholar]
- 25.Oliver, C. L., Miranda, M. B., Shangary, S., Land, S., Wang, S., and Johnson, D. E. (2005) Mol. Cancer Ther. 4 23-31 [PubMed] [Google Scholar]
- 26.Oltersdorf, T., Elmore, S. W., Shoemaker, A. R., Armstrong, R. C., Augeri, D. J., Belli, B. A., Bruncko, M., Deckwerth, T. L., Dinges, J., Hajduk, P. J., Joseph, M. K., Kitada, S., Korsmeyer, S. J., Kunzer, A. R., Letai, A., Li, C., Mitten, M. J., Nettesheim, D. G., Ng, S., Nimmer, P. M., O'connor, J. M., Oleksijew, A., Petros, A. M., Reed, J. C., Shen, W., Tahir, S. K., Thompson, C. B., Tomaselli, K. J., Wang, B., Wendt, M. D., Zhang, H., Fesik, S. W., and Rosenberg, S. H. (2005) Nature 435 677-681 [DOI] [PubMed] [Google Scholar]
- 27.Lickliter, J. D., Wood, N. J., Johnson, L., McHugh, G., Tan, J., Wood, F., Cox, J., and Wickham, N. W. (2003) Leukemia 17 2074-2080 [DOI] [PubMed] [Google Scholar]
- 28.Kitada, S., Leone, M., Sareth, S., Zhai, D., Reed, J. C., and Pellecchia, M. (2003) J. Med. Chem. 46 4259-4264 [DOI] [PubMed] [Google Scholar]
- 29.Real, P. J., Cao, Y., Wang, R., Nikolovska-Coleska, Z., Sanz-Ortiz, J., Wang, S., and Fernandez-Luna, J. L. (2004) Cancer Res. 64 7947-7953 [DOI] [PubMed] [Google Scholar]
- 30.Zhai, D., Jin, C., Satterthwait, A. C., and Reed, J. C. (2006) Cell Death Differ. 13 1419-1421 [DOI] [PubMed] [Google Scholar]
- 31.Chao, D. T., and Korsmeyer, S. J. (1998) Annu. Rev. Immunol. 16 395-419 [DOI] [PubMed] [Google Scholar]
- 32.Crompton, M., Virji, S., Doyle, V., Johnson, N., and Ward, J. M. (1999) Biochem. Soc. Symp. 66 167-179 [DOI] [PubMed] [Google Scholar]
- 33.Kuwana, T., Bouchier-Hayes, L., Chipuk, J. E., Bonzon, C., Sullivan, B. A., Green, D. R., and Newmeyer, D. D. (2005) Mol. Cell 17 525-535 [DOI] [PubMed] [Google Scholar]
- 34.Nakagawa, T., Shimizu, S., Watanabe, T., Yamaguchi, O., Otsu, K., Yamagata, H., Inohara, H., Kubo, T., and Tsujimoto, Y. (2005) Nature 434 652-658 [DOI] [PubMed] [Google Scholar]
- 35.Baines, C. P., Kaiser, R. A., Purcell, N. H., Blair, N. S., Osinska, H., Hambleton, M. A., Brunskill, E. W., Sayen, M. R., Gottlieb, R. A., Dorn, G. W., Robbins, J., and Molkentin, J. D. (2005) Nature 434 658-662 [DOI] [PubMed] [Google Scholar]
- 36.Rovere-Querini, P., Capobianco, A., Scaffidi, P., Valentinis, B., Catalanotti, F., Giazzon, M., Dumitriu, I. E., Muller, S., Iannacone, M., Traversari, C., Bianchi, M. E., and Manfredi, A. A. (2004) EMBO Rep. 5 825-830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang, Y. H., Bhunia, A., Wan, K. F., Lee, M. C., Chan, S. L., Yu, V. C., and Mok, Y. K. (2006) J. Mol. Biol. 364 536-549 [DOI] [PubMed] [Google Scholar]
- 38.Kabeya, Y., Mizushima, N., Ueno, T., Yamamoto, A., Kirisako, T., Noda, T., Kominami, E., Ohsumi, Y., and Yoshimori, T. (2000) EMBO J. 19 5720-5728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marzo, I., Brenner, C., Zamzami, N., Susin, S. A., Beutner, G., Brdiczka, D., Remy, R., Xie, Z. H., Reed, J. C., and Kroemer, G. (1998) J. Exp. Med. 187 1261-1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shimizu, S., Konishi, A., Kodama, T., and Tsujimoto, Y. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 3100-3105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Susin, S. A., Zamzami, N., Castedo, M., Daugas, E., Wang, H. G., Geley, S., Fassy, F., Reed, J. C., and Kroemer, G. (1997) J. Exp. Med. 186 25-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shimizu, S., Eguchi, Y., Kamiike, W., Funahashi, Y., Mignon, A., Lacronique, V., Matsuda, H., and Tsujimoto, Y. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 1455-1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mizuta, T., Shimizu, S., Matsuoka, Y., Nakagawa, T., and Tsujimoto, Y. (2007) J. Biol. Chem. 282 16623-16630 [DOI] [PubMed] [Google Scholar]
- 44.Lei, X., Chen, Y., Du, G., Yu, W., Wang, X., Qu, H., Xia, B., He, H., Mao, J., Zong, W., Liao, X., Mehrpour, M., Hao, X., and Chen, Q. (2006) FASEB J. 20 2147-2149 [DOI] [PubMed] [Google Scholar]
- 45.Eskes, R., Antonsson, B., Osen-Sand, A., Montessuit, S., Richter, C., Sadoul, R., Mazzei, G., Nichols, A., and Martinou, J. C. (1998) J. Cell Biol. 143 217-224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Delft, M. F., Wei, A. H., Mason, K. D., Vandenberg, C. J., Chen, L., Czabotar, P. E., Willis, S. N., Scott, C. L., Day, C. L., Cory, S., Adams, J. M., Roberts, A. W., and Huang, D. C. (2006) Cancer Cell 10 389-399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Milanesi, E., Costantini, P., Gambalunga, A., Colonna, R., Petronilli, V., Cabrelle, A., Semenzato, G., Cesura, A. M., Pinard, E., and Bernardi, P. (2006) J. Biol. Chem. 281 10066-10072 [DOI] [PubMed] [Google Scholar]
- 48.Wang, J., Wei, Q., Wang, C. Y., Hill, W. D., Hess, D. C., and Dong, Z. (2004) J. Biol. Chem. 279 19948-19954 [DOI] [PubMed] [Google Scholar]
- 49.Armstrong, J. S., Steinauer, K. K., French, J., Killoran, P. L., Walleczek, J., Kochanski, J., and Knox, S. J. (2001) Exp. Cell Res. 262 170-179 [DOI] [PubMed] [Google Scholar]
- 50.Degenhardt, K., Chen, G., Lindsten, T., and White, E. (2002) Cancer Cell 2 193-203 [DOI] [PubMed] [Google Scholar]
- 51.Chmura, S. J., Dolan, M. E., Cha, A., Mauceri, H. J., Kufe, D. W., and Weichselbaum, R. R. (2000) Clin. Cancer Res. 6 737-742 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.