Abstract
An ATP binding cassette transporter LolCDE complex releases lipoproteins from the inner membrane of Escherichia coli in an ATP-dependent manner, leading to the formation of a complex between a lipoprotein and a periplasmic chaperone, LolA. LolA is proposed to undergo a conformational change upon the lipoprotein binding. The lipoprotein is then transferred from the LolA-lipoprotein complex to the outer membrane via LolB. Unlike most ATP binding cassette transporters mediating the transmembrane flux of substrates, the LolCDE complex catalyzes the extrusion of lipoproteins anchored to the outer leaflet of the inner membrane. Moreover, the LolCDE complex is unique in that it can be purified as a liganded form, which is an intermediate of the lipoprotein release reaction. Taking advantage of these unique properties, we established an assay system that enabled the analysis of a single cycle of lipoprotein transfer reaction from liganded LolCDE to LolA in a detergent solution. The LolA-lipoprotein complex thus formed was physiologically functional and delivered lipoproteins to the outer membrane in a LolB-dependent manner. Vanadate, a potent inhibitor of the lipoprotein release from proteoliposomes, was found to inhibit the release of ADP from LolCDE. However, a single cycle of lipoprotein transfer occurred from vanadate-treated LolCDE to LolA, indicating that vanadate traps LolCDE at the energized state.
Bacterial lipoproteins possess Cys at the N terminus, which is covalently modified with thioether-linked diacylglycerol, and an amino-linked acyl chain and are anchored to membranes through three acyl chains (1). Lipoproteins synthesized as precursors in the cytoplasm are translocated across the inner membrane by a Sec apparatus and then processed to the mature forms on the periplasmic leaflet of the inner membrane (2, 3). Most lipoproteins in Escherichia coli are targeted to the outer membrane, whereas some are localized in the inner membrane (4). The residue at position 2 determines the membrane specificity; Asp is a general inner membrane retention signal, whereas other residues direct lipoproteins to the outer membrane (5–7).
The Lol system composed of five proteins catalyzes the sorting of lipoproteins to the outer membrane (8). The LolCDE complex belongs to the ATP binding cassette (ABC)2 transporter superfamily and is composed of one molecule each of membrane subunits LolC and LolE and a homodimer of nucleotide binding subunit LolD. LolCDE releases outer membrane-specific lipoproteins from the inner membrane, resulting in the formation of a complex between the released lipoproteins and LolA, a periplasmic molecular chaperone. Although lipoproteins are highly hydrophobic because of their N-terminal acyl chains, the LolA-lipoprotein complex is hydrophilic and reaches the lipoprotein receptor LolB in the outer membrane by crossing the hydrophilic periplasm. LolB is itself a lipoprotein and mediates the transfer of lipoproteins from LolA to the outer membrane (9–11). The structures of LolA and LolB are very similar to each other; both have an incomplete β-barrel covered with an α-helical lid, a hydrophobic cavity thereby being formed which is most likely the binding site of the acyl chains of lipoproteins (12). The hydrophobic cavity of LolB is open to the external milieu, whereas that of LolA is closed. It is speculated that the LolA lid undergoes opening and closing upon the binding and release of lipoproteins, respectively. The lid opening is presumably coupled to ATP hydrolysis by LolCDE at the cytoplasmic side of the inner membrane (12). Thus, ATP energy is most likely transmitted to the periplasmic LolA from the cytoplasm through LolCDE.
Molecular events coupled to substrate transport have been examined in detail for various ABC transporters (13). Maltose importer MalFGK2 functions together with a periplasmic maltose-binding protein (MBP). MBP binds maltose at high affinity in the periplasm and then interacts with MalFGK2 in the inner membrane, causing the stimulation of ATP hydrolysis by MalFGK2 at the cytoplasmic side of the inner membrane. The phosphate analogue vanadate has been found to stabilize the transition states of ABC transporters such as P-glycoprotein (14) and MalFGK2 (15). It was found that MBP tightly associates with MalFGK2 but no longer binds maltose in the transition state (16, 17). Vanadate is trapped together with ADP in this transition state of MalFGK2. It has been thought that the transition state of MalFGK2 represents an intermediate of maltose transport (16).
The LolCDE complex can be purified with tightly associated outer membrane-specific lipoproteins (18). The liganded LolCDE represents an intermediate of the lipoprotein transfer reaction formed in the inner membrane. Taking advantage of this unique property of LolCDE, the molecular events coupled to ATP binding and hydrolysis were examined in detail.
EXPERIMENTAL PROCEDURES
Materials—DDM was purchased from Dojindo Laboratories, Kumamoto, Japan. TALON resin was from Clontech. FLAG M2 affinity resin and FLAG peptides were products of Sigma. Antibodies against LolA (9), Pal (19), Lpp (20), and Lol-CDE subunits (21) were raised in rabbits as described.
Preparation of Liganded LolCDE—The method reported by Ito et al. (18) was slightly modified. E. coli JM83 cells (22) harboring pKM402 carrying lolC and lolD-his under PBAD and pKM301 carrying lolE under tacPO were grown on Luria broth supplemented with 50 μg/ml ampicillin and 25 μg/ml chloramphenicol at 30 °C. Where specified, the strain harbored derivatives of pKM402 carrying the gene for LolD(E171Q) as reported (18). When the absorbance at 660 nm reached 0.8, LolC and LolD were induced with 0.2% arabinose. After 2 h, LolE was induced with 1 mm isopropyl-β-d-thiogalactopyranoside for 1 h. A membrane fraction (100 mg) was prepared from LolCDE-overproducing cells and then solubilized with 50 ml of 50 mm Tris-HCl (pH 7.5) containing 1% DDM, 5 mm MgSO4, and 10% glycerol for 30 min on ice. A supernatant was obtained by centrifugation at 100,000 × g for 40 min and then applied on a 2-ml TALON column that had been equilibrated with buffer A (50 mm Tris-HCl (pH 7.5), 300 mm NaCl, 0.01% DDM, and 10% glycerol). The column was washed with 60 ml of buffer A supplemented with 10 mm imidazole, and LolCDE was eluted with a linear gradient of imidazole (10 to 250 mm) in buffer A. LolCDE was obtained in the fractions corresponding to 60 mm imidazole. The fractions containing LolCDE were dialyzed against 50 mm Tris-HCl (pH 7.5) containing 0.01% DDM and 10% glycerol.
Preparation of FLAG-tagged LolA—E. coli TT016 cells (lac-lolA) (23) harboring pSW77 (24) carrying the gene for LolA-FLAG under PBAD were grown at 37 °C on Luria broth supplemented with chloramphenicol, kanamycin, and 0.1 mm isopropyl-β-d-thiogalactopyranoside, as reported (24). LolA-FLAG was induced with 0.2% arabinose when the absorbance at 660 nm reached 0.6. Periplasmic fractions containing LolA-FLAG were adsorbed to a 1-ml anti-FLAG M2 affinity column (Sigma) and eluted with 10 mm Tris-HCl (pH 8.0) containing 0.1 mg/ml FLAG peptides (Sigma). The purified LolA-FLAG was dialyzed against 20 mm Tris-HCl (pH 7.5) overnight at 4 °C and then kept frozen at -80 °C.
Dissociation of Lipoproteins from Liganded LolCDE—Liganded LolCDE (1 μg) was incubated on ice for 30 min in 10 μl of 50 mm Tris-HCl (pH 7.5) containing 5 mm MgSO4, 10% glycerol, and the specified concentrations of DDM with or without 2 mm nucleotides (20 mm for AMP-PNP) and/or 20 μg/ml LolA-FLAG. Incubation was terminated by the addition of 1 ml of 50 mm Tris-HCl (pH 7.5) containing 5 mm MgSO4, 10% glycerol, and the specified concentrations of DDM followed by treatment with 50 μl TALON resin for 10 min at 4 °C. Elution of LolCDE from the resin was performed with the above buffer supplemented with 250 mm imidazole. The resin-bound and -unbound fractions were precipitated with trichloroacetic acid and then analyzed by SDS-PAGE and Western blotting with the specified antibodies.
Vanadate Trapping—Liganded LolCDE (1 μg) was incubated on ice for 30 min in 10 μl of 50 mm Tris-HCl (pH 7.5) containing 5 mm MgSO4, 0.01% DDM, 10% glycerol, the indicated concentrations of vanadate, and either [α-32P]ATP or [γ-32P]ATP (0.37 MBq, 925 GBq/mmol). The reaction was terminated by dilution with 1 ml of the above buffer. The radioactivity in the LolCDE fraction was determined with a scintillation counter after adsorption to TALON resin. A stock solution of 200 mm Na3VO4 was prepared as reported (25). This solution was boiled, and its pH was readjusted immediately before use as reported (25).
Preparation of the Homogeneous LolCDE-Pal Complex—Pal was overproduced from a plasmid, pSS4-1, together with LolCDE in E. coli HMS174(DE3) cells (Novagen) harboring pKM402 and pKM301, as reported (18). Liganded LolCDE was purified on a TALON affinity column as described above and then on an anion exchange column of Mono Q (GE Healthcare) which had been equilibrated with 50 mm Tris-HCl (pH 7.5) containing 10% glycerol and 0.01% DDM. LolCDE exclusively containing Pal was purified with a linear gradient (0.1–0.5 m) of NaCl as reported (18).
Other Methods—Outer membranes were prepared by sucrose density gradient (25–55%) centrifugation as reported (9) from E. coli JE5505 (lpp) (26) and SM704 (lpp lac-lolB) (27) cells and confirmed by the presence of OmpA and absence of SecG. To deplete LolB, SM704 was grown on Luria broth for 5 h in the absence of isopropyl-β-d-thiogalactopyranoside. SDS-PAGE analysis (28) was performed as reported. Densitometric quantification was performed with an ATTO Densitograph.
RESULTS
LolA-dependent Dissociation of Lipoproteins from LolCDE—When the solubilization and purification of LolCDE were performed with 1% DDM in the absence of ATP, the isolated LolCDE contained various outer membrane-specific but not inner membrane-specific lipoproteins (18). The liganded LolCDE thus isolated can be kept soluble in 0.01% DDM, which is slightly higher than its critical micellar concentration (0.0087%), as reported for MalFGK2 (29). It was then revealed that ATP binding to LolCDE decreases the strength of the hydrophobic interaction between LolCDE and lipoproteins and, therefore, causes the dissociation of lipoproteins from LolCDE in the presence of 1% DDM. In contrast, when liganded LolCDE was isolated, it remained liganded in the presence of 0.01% DDM even after the addition of ATP (18). Because the incubation of LolCDE at 30 °C causes inactivation in the absence of phospholipids (30), the following experiments were performed on ice.
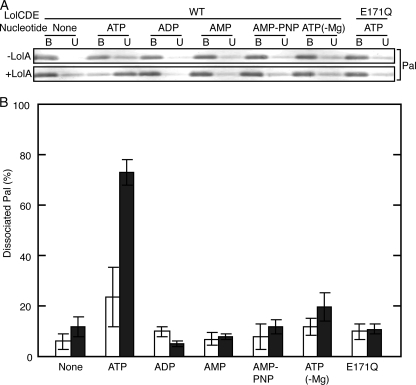
Liganded LolCDE with His-tagged LolD was incubated in 0.01% DDM containing various nucleotides in the presence and absence of LolA followed by adsorption to a TALON affinity column. The TALON-bound and -unbound fractions were examined by SDS-PAGE and Western blotting with anti-Pal antibodies (Fig. 1A). The relative amounts of Pal dissociated under the respective conditions were then determined (Fig. 1B). Significant dissociation of Pal occurred only when both LolA and ATP were present. On the other hand, ADP, AMP, AMP-PNP, or ATP without Mg2+ did not cause the dissociation of Pal irrespective of the presence or absence of LolA. LolD carrying the E171Q mutation can bind but cannot hydrolyze ATP (18). The LolCD(E171Q)E complex remained liganded after the addition of ATP even in the presence of LolA. These results indicate that ATP hydrolysis is essential for the LolA-dependent dissociation of Pal from liganded LolCDE. The requirement for LolA strongly suggested that the dissociation of Pal observed here mimics the in vivo lipoprotein transfer reaction.
FIGURE 1.
Dissociation of lipoproteins from liganded LolCDE. Liganded LolCDE (1 μg) containing His-tagged LolD was incubated in 10 μl of 50 mm Tris-HCl (pH 7.5) containing 5 mm MgSO4, 0.01% DDM with or without 2 mm nucleotides (20 mm for AMP-PNP) and LolA-FLAG (20 μg/ml) on ice for 30 min. Dissociation of Pal from liganded LolCDE was examined as described under “Experimental Procedures.” Where specified, LolCDE contained the LolD(E171Q) mutant, and ATP was added in the absence of Mg2+. A, Pal in the TALON-bound (B) and -unbound (U) fractions was detected by SDS-PAGE and Western blotting with anti-Pal antibodies. B, the experiments shown in A were repeated three times, and the amounts of Pal in the TALON-bound and -unbound fractions were quantitated. The percentages of Pal in the TALON-unbound fractions are shown with error bars. Closed bars, +LolA; open bars, -LolA. WT, wild type.
The LolA- and ATP-dependent dissociation of Pal also occurred in the presence of 1% n-heptyl-β-d-thioglucopyranoside (critical micellar concentration, 0.88%) as was the case of 0.01% DDM. On the other hand, 0.2% sucrose monocaprate (critical micellar concentration, 0.125%) or 1% n-octyl-β-d-glucopyranoside (critical micellar concentration, 0.73%) caused LolA-independent dissociation of Pal.3 We previously reconstituted liganded LolCDE into proteoliposomes using 1.2% sucrose monocaprate (18). It seems highly likely that lipoproteins were dissociated from LolCDE and separately reconstituted into proteoliposomes in our previous reconstitution experiments.
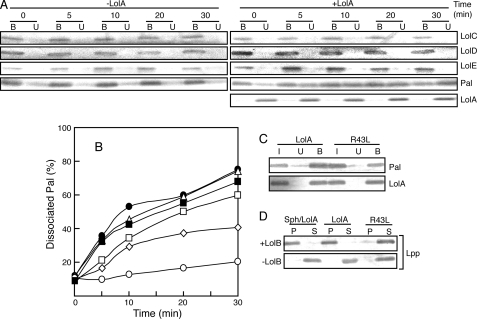
Transfer of Pal from LolCDE to LolA—The time course of ATP-dependent dissociation of Pal from LolCDE was examined in the presence of various concentrations of LolA. At specified times the TALON-bound and -unbound fractions were examined by SDS-PAGE and Western blotting with the indicated antibodies. The results obtained with or without 20 μg/ml LolA are shown in Fig. 2A. LolC, LolD, and LolE were always detected in the bound fraction, whereas LolA was recovered in the unbound fraction (Fig. 2A). A significant amount of Pal was dissociated from LolCDE and recovered in the unbound fraction only in the presence of LolA. The dissociation of Pal examined in the presence of various concentrations of LolA was quantified and plotted as a function of time (Fig. 2B). The rate of dissociation increased with the increase in the concentration of LolA up to 15∼20 μg/ml.
FIGURE 2.
Pal was released as a complex with LolA and incorporated into outer membranes via LolB. LolA-dependent dissociation of Pal from liganded LolCDE was examined on ice for the specified times in the presence of 0.01% DDM, 2 mm ATP, 5 mm MgSO4, and various concentrations of LolA-FLAG as in Fig. 1. A, the assays were performed with 20 μg/ml LolA-FLAG or no LolA. The TALON-bound (B) and -unbound (U) fractions were analyzed by SDS-PAGE and immunoblotting with the specified antibodies. B, the amounts of Pal released in the presence of various concentrations of LolA-FLAG were determined after immunoblotting as described in Fig. 1. The concentrations of LolA-FLAG were 0 (open circles), 5 (open diamonds), 10 (open squares), 15 (open triangles), and 20 (closed circles) μg/ml. The assay was also performed with 20 μg/ml LolA(R43L) (closed squares). C, lipoproteins were dissociated from liganded LolCDE in the presence of 20 μg/ml LolA-FLAG or LolA(R43L)-FLAG as in Fig. 1. TALON-unbound fractions containing dissociated lipoproteins were then applied to the FLAG affinity column. Pal and LolA in the FLAG column-bound (B) and -unbound (U) fractions together with input material (I) were analyzed by SDS-PAGE and Western blotting with the respective antibodies as described under “Experimental Procedures.” D, Lpp was released from liganded LolCDE as a complex with LolA-FLAG or LolA(R43L)-FLAG as shown in C and then incubated with 0. 2 mg/ml outer membranes containing or not containing LolB for 30 min at 30 °C as reported (27). After centrifugation, membranes (P) and supernatants (S) were analyzed by SDS-PAGE and immunoblotting with anti-Lpp antibodies. As a control, Lpp was released from spheroplasts prepared from 5 × 108 cells of E. coli MC4100 by the addition of 20 μg/ml LolA-FLAG (Sph/LolA) and then subjected to the outer membrane incorporation assay.
To determine whether or not Pal was dissociated from LolCDE as a complex with LolA, FLAG-tagged LolA or its R43L derivative was used for the ATP-dependent dissociation in the presence of 0.01% DDM. TALON-unbound fractions containing Pal were then applied to a FLAG affinity column (Fig. 2C). Pal bound to the FLAG affinity column with not only LolA but also the LolA(R43L) derivative. The LolA(R43L) mutant can bind a lipoprotein but cannot transfer it to LolB (23). These results indicate that Pal was released from the liganded LolCDE as a complex with LolA or LolA(R43L). It was shown previously that a single molecule of LolCDE binds to a single molecule of a lipoprotein (18). The dissociation of Pal observed here, therefore, represents a single cycle of Pal transfer. The lipoproteins bound to the FLAG affinity column as a complex with LolA or LolA(R43L) were eluted with FLAG peptides in the absence of DDM.
We next examined whether or not the LolA-lipoprotein complex isolated as described above can incorporate lipoproteins into outer membranes in a LolB-dependent manner. Because the outer membrane contained endogenous Pal, we attempted to construct a Δpal-ΔlolB strain. However, the strain could not be constructed by unknown reasons. We, therefore, examined incorporation of the major outer membrane lipoprotein Lpp into outer membranes lacking Lpp (Fig. 2D). When the release reaction was performed with LolA and spheroplasts, Lpp was incorporated into the outer membrane in a LolB-dependent manner. The LolA-lipoprotein complex formed in vitro also incorporated Lpp into the LolB-containing outer membrane. In contrast, the R43L-lipoprotein complex did not incorporate Lpp into the outer membrane because R43L is unable to transfer lipoproteins to LolB (23). Taken together, these results indicate that the LolA-lipoprotein complex formed from the liganded LolCDE is a physiological complex.
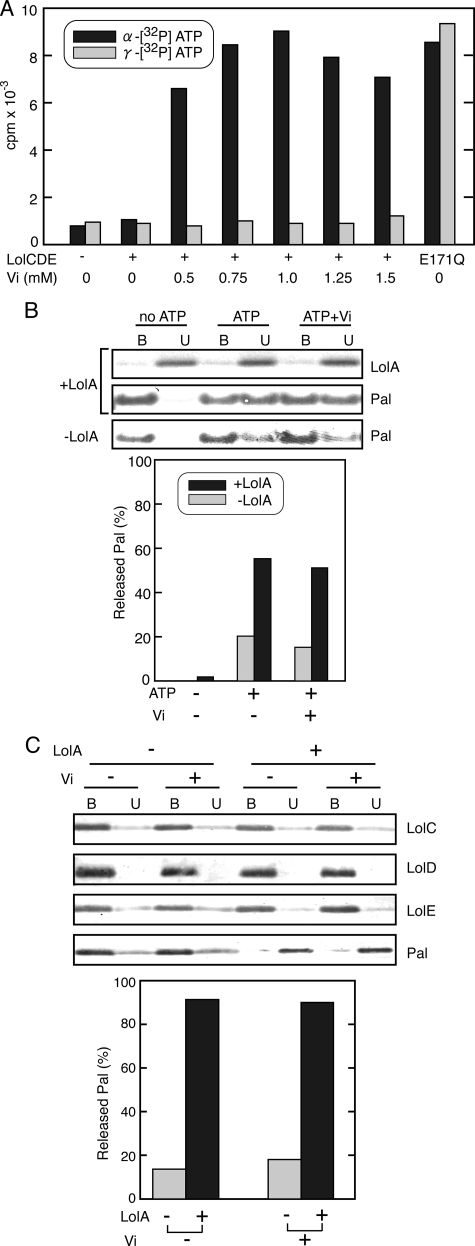
Vanadate Does Not Inhibit a Single Cycle of Lipoprotein Transfer—Vanadate is a phosphate analogue and inhibits ABC transporters (15). It has been reported that vanadate stabilizes maltose permease MalFGK2 in a transition state in which MBP tightly binds to MalFGK2 with vanadate and ADP. Lipoprotein release from reconstituted proteoliposomes by LolCDE is also sensitive to vanadate (27, 31). We examined whether or not ADP is trapped in vanadate-treated LolCDE as reported for MalFGK2 (Fig. 3). LolCDE was incubated with either α-or γ-labeled [32P]ATP and various concentrations of vanadate for 30 min on ice and then adsorbed to a metal affinity column. The ATPase activity of LolCDE was 1.7 mol of ATP hydrolyzed/min/mol of LolCDE under these conditions. When γ-labeled ATP was used, essentially no label was detected in LolCDE except for in the LolCD(E171Q)E mutant irrespective of the presence or absence of vanadate (Fig. 3A). LolCD(E171Q)E was labeled with both [α-32P]ATP and [γ-32P]ATP because it can bind but cannot hydrolyze ATP (18). In contrast, when LolCDE was labeled with α-[32P]ATP, vanadate-dependent labeling occurred. For maximum labeling, about 1 mm vanadate was required. More than 90% of the ATPase activity was inhibited by 1 mm vanadate. These results indicate that vanadate traps ADP generated through ATP hydrolysis in the LolCDE complex. The amount of ADP associated with 1 mol of the LolCDE complex was about 0.9 mol. We then examined a single cycle of Pal transfer from liganded LolCDE to LolA in the presence of ATP and vanadate (Fig. 3B). Vanadate had no inhibitory effect on the transfer of Pal. Strikingly, LolA added after complete inhibition of ATP hydrolysis by vanadate also caused the release of Pal (Fig. 3C), whereas ADP remained trapped in LolCDE even after LolA addition (data not shown). LolC and LolE remained bound to TALON with LolD, indicating that the LolCDE complex is intact under these conditions. However, when vanadate-treated LolCDE was isolated, certain amounts of LolC and LolE were recovered in the TALON-unbound fraction (data not shown), indicating that treatment with vanadate decreases the stability of the LolCDE complex. Nevertheless, an appreciable amount of Pal was released from isolated vanadate-treated LolCDE by the addition of LolA. Taken together, these results indicate that vanadate-treated LolCDE is able to transfer lipoproteins to LolA and concomitantly induce opening of the LolA lid, although it is unable to recycle the lipoprotein release reaction (27, 31).
FIGURE 3.
Vanadate fixes LolCDE in a transition state. A, liganded LolCDE or LolCD(E171Q)E was incubated on ice with either [α-32P]ATP or [γ-32P]ATP in the presence of the specified concentrations of vanadate (Vi). Radioactivity retained by LolCDE was determined after adsorption to TALON resin as described under “Experimental Procedures.” B, dissociation of Pal was examined in the presence and absence of 1 mm vanadate, 20 μg/ml LolA-FLAG, and 2 mm ATP, as indicated. The TALON-bound (B) and -unbound (U) fractions were then analyzed by SDS-PAGE and Western blotting (upper panel) and quantified to determine the relative amount of Pal released into the unbound fraction (lower panel). C, liganded LolCDE was incubated with 2 mm ATP and 1 mm vanadate on ice for 30 min. LolA-FLAG was then added or not added to the reaction mixture, which then stood for 30 min. The TALON-bound (B) and -unbound (U) fractions were analyzed by SDS-PAGE and immunoblotting with the specified antibodies and quantified as in Fig. 2B (upper panel).
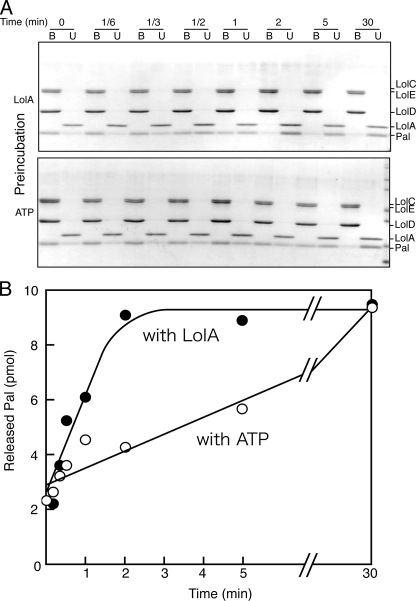
Acceleration of the in Vitro Lipoprotein Transfer Reaction—Although the lipoprotein transfer from liganded LolCDE to LolA is a single cycle reaction, it took almost 30 min on ice (Fig. 2). The liganded LolCDE examined above contained various outer membrane-specific lipoproteins (18). To kinetically reveal why the transfer reaction is slow, the homogeneous LolCDE-Pal complex was isolated and used for the transfer reaction. One molecule of this complex contained one molecule of Pal (18). The complex was first incubated with either LolA or ATP, and then the transfer reaction was started by the addition of the other. TALON-bound and -unbound fractions were analyzed by SDS-PAGE and Coomassie Brilliant Blue staining (Fig. 4A). The amount of Pal released from LolCDE was determined and plotted as a function of time (Fig. 4B). When the LolCDE-Pal complex was preincubated with LolA, the transfer reaction started by the addition of ATP was greatly stimulated and completed within 2 min even on ice. The LolA concentration (20 μg/ml) was found to be saturating (data not shown). On the other hand, preincubation with ATP did not cause a rapid transfer reaction. However, it should be pointed out that the rate of ATP hydrolysis was not affected by preincubation with LolA (data not shown), indicating that ATP hydrolysis and Pal release are not tightly coupled. Taken together, these results indicate that the interaction between LolA and the LolCDE-Pal complex is responsible for a slow reaction.
FIGURE 4.
Preincubation with LolA accelerates the lipoprotein transfer. The LolCDE-Pal complex (10 pmol, 1.6 μg) was preincubated on ice for 5 min with either 20 μg/ml LolA-FLAG (12 pmol) or 2 mm ATP. The release of Pal was then induced by the addition of ATP or LolA-FLAG to the respective preincubated mixture. A, the release of Pal was analyzed at the indicated times by SDS-PAGE and Coomassie Brilliant Blue-staining after adsorption to TALON resin. B and U represent the TALON-bound and -unbound fractions, respectively. B, the amounts of Pal in the TALON-bound and -unbound fractions shown in A were densitometrically quantified and are plotted as a function of time. The results are for experiments performed after preincubation with LolA (closed circles) or ATP (open circles).
DISCUSSION
Our previous observations both published (31) and unpublished4 indicate that the formation of the LolA-lipoprotein complex strictly depends on ATP hydrolysis by LolCDE. The complex is formed when lipoproteins are released from spheroplasts on the addition of LolA (9), whereas it was not formed when lipoproteins dissolved in a detergent were rapidly diluted with a solution containing LolA or dialyzed with LolA against a solution containing no detergent or when LolA was denatured and re-natured in the presence of lipoproteins.4 Based on these biochemical and crystallographic (12) observations, it has been speculated that LolCDE utilizes ATP energy for not only membrane detachment of lipoproteins but also opening of the LolA lid (12).
We established here the experimental conditions under which lipoproteins bound to LolCDE are transferred to LolA at near 100% efficiency in an ATP-dependent manner (Fig. 4). The LolA-lipoprotein complex thus formed was physiologically functional and delivered lipoproteins to the outer membrane in a LolB-dependent manner (Fig. 2C). From these results, we concluded that the lipoprotein transfer reaction from liganded LolCDE to LolA in the presence of 0.01% DDM mimics the in vivo reaction in the inner membrane. Because this novel assay system does not involve reconstitution of LolCDE into proteoliposomes, the molecular events coupled to a single cycle of lipoprotein transfer could be examined in detail. Although the effects of phospholipids on the sorting of lipoproteins cannot be examined unless LolCDE is reconstituted into proteoliposomes (32), the current assay system is superior to reconstitution in the efficiency of the transfer reaction because only about 10% of lipoproteins are released from reconstituted proteoliposomes (31). Sucrose monocaprate was used to reconstitute LolCDE into proteoliposomes (31). However, this detergent was recently found to dissociate lipoproteins from liganded LolCDE.3 Therefore, a single cycle of lipoprotein transfer from liganded LolCDE cannot be examined in reconstituted proteoliposomes. Moreover, multiple cycles of the transfer reaction take place in proteoliposomes reconstituted with unliganded LolCDE and lipoproteins (33), which are reconstituted in different orientations. Dissection of molecular events underlying the transfer reaction is, therefore, difficult in proteoliposomes. In contrast, the lipoprotein transfer from liganded LolCDE is a single cycle and, thus, can be dissected, as shown here.
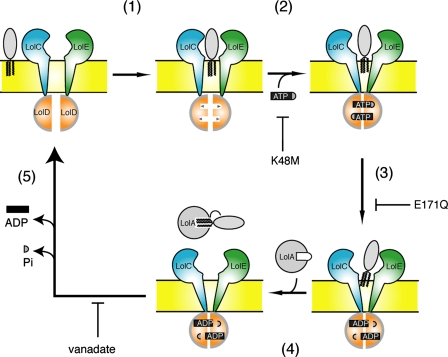
Based on biochemical, structural, and genetic data obtained for different ABC transporters, “the ATP switch model” was proposed for ABC transporters by Higgins and Linton (13). In this model ATP binding and ATP hydrolysis, respectively, induce the formation and dissociation of a dimer of the nucleotide binding subunit, thereby providing a regulated switch that induces conformational changes in the transmembrane subunit. The crystal structures of bacterial ABC exporters recently revealed an outward-facing conformation of ATP-bound transporters, whereas ATP hydrolysis induces an inward-facing conformation (34). Moreover, structure of MalFGK2 in complex with MBP was very recently solved (17). We previously reported that the high affinity binding of lipoprotein to LolC/E initiates the lipoprotein release reaction in the inner membrane and causes an increase in the affinity of LolD for ATP (step 1 in Fig. 5). ATP binding to LolD then decreases the affinity of LolC/E for lipoproteins (step 2), thereby causing dissociation of liganded LolCDE in the presence of 1% DDM. Here we found that ATP hydrolysis further weakens the interaction of LolCDE with lipoproteins, although the lipoproteins remain associated with LolCDE (step 3). The addition of LolA then triggers the transfer of lipoproteins from LolCDE and at the same time opening of the LolA lid (step 4). Release of inorganic phosphate and ADP, presumably in this order as speculated (35), from LolD allows recovery of the initial conformation of LolCDE required for a new cycle of transfer reaction (step 5). The crystal structure of MJ0796, a methanococcal LolD homolog exhibiting 43.7% sequence identity, showed a very similar tertiary fold to those of the ATPase subunits of other ABC transporters and contained two nucleotides (36, 37). It seems likely that the binding of two ATP molecules to LolD is cooperative, although we have no direct evidence for this. The most important finding here is that vanadate-trapped LolCDE can transfer lipoproteins to LolA, which concomitantly undergoes a conformational change (step 4). On the other hand, AMP-PNP does not induce the lipoprotein transfer from liganded LolCDE. Therefore, the vanadate-trapped LolCDE exists at the “energized state,” which is brought about by ATP hydrolysis (step 3). We, thus, established the conditions under which a single cycle of lipoprotein transfer takes place from liganded LolCDE to LolA in a detergent solution.
FIGURE 5.
Molecular events involved in the LolCDE-dependent transfer of lipoproteins from the inner membrane to LolA. Based on the results shown here and those reported by Ito et al. (18), the detailed mechanisms underlying membrane detachment of lipoproteins are depicted. For more information, see “Results.” Mutations K48M and E171Q in LolD inhibit the specified steps of the reaction (18). Vanadate inhibits step 5.
We recently succeeded in reconstituting the LolCDE complex from separately isolated subunits (30). Our system is expected to be useful for elucidation of the roles of the respective subunits in the lipoprotein transfer reaction. Indeed, the active complex was reconstituted from LolE and LolD without LolC (30), although both LolC and LolE are essential for the growth of E. coli (21). The results shown here and previously reported ones seem to be important for clarifying the roles of each subunit of the LolCDE complex in the transfer and utilization of ATP energy for the retraction of lipoproteins and the conformational change of LolA.
This work was supported by grants from the Ministry of Education, Science, Sports, and Culture of Japan (to H. T.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: ABC, ATP binding cassette; AMP-PNP, adenosine 5′-[β-γ-imido]triphosphate; DDM, n-dodecyl-β-D-maltopyranoside; MBP, maltose-binding protein; sucrose monocaprate, β-d-fructopyranosyl-α-d-glucopyranoside monodecanoate.
N. Taniguchi and H. Tokuda, unpublished observation.
N. Yokota and H. Tokuda, unpublished information.
References
- 1.Sankaran, K., and Wu, H. C. (1994) J. Biol. Chem. 269 19701-19706 [PubMed] [Google Scholar]
- 2.Hayashi, S., and Wu, H. C. (1990) J. Bioenerg. Biomembr. 22 451-471 [DOI] [PubMed] [Google Scholar]
- 3.Pugsley, A. P. (1993) Microbiol. Rev. 57 50-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tokuda, H., Matsuyama, S., and Tanaka-Masuda, K. (2007) in The Periplasm (Ehrmann, M., ed) pp. 67-79, American Society for Microbiology, Washington, DC
- 5.Yamaguchi, K., Yu, F., and Inouye, M. (1988) Cell 53 423-432 [DOI] [PubMed] [Google Scholar]
- 6.Seydel, A., Gounon, P., and Pugsley, A. P. (1999) Mol. Microbiol. 34 810-821 [DOI] [PubMed] [Google Scholar]
- 7.Terada, M., Kuroda, T., Matsuyama, S., and Tokuda, H. (2001) J. Biol. Chem. 276 47690-47694 [DOI] [PubMed] [Google Scholar]
- 8.Tokuda, H., and Matsuyama, S. (2004) Biochim. Biophys. Acta 1693 5-13 [DOI] [PubMed] [Google Scholar]
- 9.Matsuyama, S., Tajima, T., and Tokuda, H. (1995) EMBO J. 14 3365-3372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuyama, S., Yokota, N., and Tokuda, H. (1997) EMBO J. 16 6847-6955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yokota, N., Kuroda, T., Matsuyama, S., and Tokuda, H. (1999) J. Biol. Chem. 274 30995-30999 [DOI] [PubMed] [Google Scholar]
- 12.Takeda, K., Miyatake, H., Yokota, N., Matsuyama, S., Tokuda, H., and Miki, K. (2003) EMBO J. 22 3199-3209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins, C. F., and Linton, K. J. (2004) Nat. Struct. Mol. Biol. 11 918-926 [DOI] [PubMed] [Google Scholar]
- 14.Urbatsch, I. L., Sankaran, B., Weber, J., and Senior, A. E. (1995) J. Biol. Chem. 270 19383-19390 [DOI] [PubMed] [Google Scholar]
- 15.Davidson, A. L., and Chen, J. (2004) Annu. Rev. Biochem. 73 241-268 [DOI] [PubMed] [Google Scholar]
- 16.Chen, J., Sharma, S., Quiocho, F. A., and Davidson, A. L. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 1525-1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oldham, M. L., Khare, D., Quiocho, F. A., Davidson, A. L., and Chen, J. (2007) Nature 450 515-521 [DOI] [PubMed] [Google Scholar]
- 18.Ito, Y., Kanamaru, K., Taniguchi, N., Miyamoto, S., and Tokuda, H. (2006) Mol. Microbiol. 62 1064-1075 [DOI] [PubMed] [Google Scholar]
- 19.Tajima, T., Yokota, N., Matsuyama, S., and Tokuda, H. (1998) FEBS Lett. 439 51-54 [DOI] [PubMed] [Google Scholar]
- 20.Hussain, M., Ichihara, S., and Mizushima, S. (1980) J. Biol. Chem. 255 3707-3712 [PubMed] [Google Scholar]
- 21.Narita, S., Tanaka, K., Matsuyama, S., and Tokuda, H. (2002) J. Bacteriol. 184 1417-1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yanisch-Perron, C., Vieira, J., and Messing, J. (1985) Gene (Amst.) 33 103-119 [DOI] [PubMed] [Google Scholar]
- 23.Miyamoto, A., Matsuyama, S., and Tokuda, H. (2001) Biochem. Biophys. Res. Commun. 287 1125-1128 [DOI] [PubMed] [Google Scholar]
- 24.Watanabe, S., Matsuyama, S., and Tokuda, H. (2006) J. Biol. Chem. 281 3335-3342 [DOI] [PubMed] [Google Scholar]
- 25.Goodno, C. C. (1982) Methods Enzymol. 85 116-123 [DOI] [PubMed] [Google Scholar]
- 26.Hirota, Y., Suzuki, H., Nishiyama, Y., and Yasuda, S. (1977) Proc. Natl. Acad. Sci. U. S. A. 74 1417-1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yakushi, T., Yokota, N., Matsuyama, S., and Tokuda, H. (1998) J. Biol. Chem. 273 32576-32581 [DOI] [PubMed] [Google Scholar]
- 28.Laemmli, U. K. (1970) Nature 227 680-685 [DOI] [PubMed] [Google Scholar]
- 29.Davidson, A. L., and Nikaido, H. (1991) J. Biol. Chem. 266 8946-8951 [PubMed] [Google Scholar]
- 30.Kanamaru, K., Taniguchi, N., Miyamoto, S., Narita, S., and Tokuda, H. (2007) FEBS J. 274 3034-3043 [DOI] [PubMed] [Google Scholar]
- 31.Yakushi, T., Masuda, K., Narita, S., Matsuyama, S., and Tokuda, H. (2000) Nat. Cell Biol. 2 212-218 [DOI] [PubMed] [Google Scholar]
- 32.Miyamoto, S., and Tokuda, H. (2007) Biochim. Biophys. Acta 1768 1848-1854 [DOI] [PubMed] [Google Scholar]
- 33.Masuda, K., Matsuyama, S., and Tokuda, H. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 7390-7395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dawson, R. J. P., and Locher, K. P. (2006) Nature 443 180-185 [DOI] [PubMed] [Google Scholar]
- 35.Sharma, S., and Davidson, A. L. (2000) J. Bacteriol. 182 6570-6576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan, Y. R., Blecker, S., Martsinkevich, O., Millen, L., Thomas, P. J., and Hunt, J. F. (2001) J. Biol. Chem. 276 32313-32321 [DOI] [PubMed] [Google Scholar]
- 37.Smith, P. C., Karpowich, N., Millen, L., Moody, J. E., Rosen, J., Thomas, P. J., and Hunt, J. F. (2001) Mol. Cell 10 139-149 [DOI] [PMC free article] [PubMed] [Google Scholar]