Abstract
IL-18 is a proinflammatory cytokine that plays an important role in natural killer cell activation and T helper 1 (Th1) cell responses. Mast cells and basophils are major inducers and effectors of allergic inflammation. Here we show that basophils and mast cells derived by culture of bone marrow cells with IL-3 for 10 days express IL-18Rα chain and that basophils produce large amounts of IL-4 and IL-13 in response to stimulation with IL-3 and IL-18. Injection of IL-12 and IL-18 inhibits IgE production in helminth-infected wild-type mice and abolishes the capacity of their basophils to produce IL-4 and IL-13 in response to stimulation either with IL-3 and IL-18 or with FcɛR cross-linkage. By contrast, this combination of cytokines actually increases IgE levels in helminth-infected IFN-γ−/− mice and enhances IL-4 and IL-13 production by their basophils. Furthermore, injection of IL-18 alone enhances basophil production of IL-4 and histamine both in wild-type and IFN-γ−/− mice. Thus, IL-18 has the potential to stimulate basophils but, when given with IL-12, exhibits an antiallergic action in vivo.
Keywords: allergy
IL-18, originally designated as IFN-γ-inducing factor, is a pleiotropic cytokine secreted by activated macrophages and Kupffer cells (1–3). In combination with IL-12, it induces IFN-γ production by T helper 1 (Th1) cells but not by Th2 cells (4–6). IL-18 enhances Fas ligand expression on Th1 cells and natural killer (NK) cells (7, 8). The IL-18R is composed of a ligand-binding chain (IL-18Rα or IL-1R-related protein) and a signal-transducing chain (IL-18Rβ or IL-1R accessory protein-like) (9–11). Th1 cells express IL-18Rα whereas Th2 cells do not; consequently, IL-18Rα can be used as a molecular marker to distinguish Th1 from Th2 cells (6, 12). NK cells constitutively express IL-18Rα and show increased perforin-mediated NK activity in response to IL-18 (13). Importantly, IL-18 also stimulates NK cells and T cells to produce IL-13 (14).
We recently have shown that administration of IL-12 and IL-18 inhibits IgE production in helminth-infected mice in an IFN-γ-dependent manner, presenting a unique approach for the treatment of allergic disorders (15). Mast cells and basophils play a key role in allergic inflammation (16, 17). Upon cross-linkage of the high-affinity IgE receptor (FcɛR1), they release various chemical mediators such as histamine and serotonin (16–18) and a series of cytokines including IL-4 and IL-13 (19–24). For this reason, we regard it important to examine the effect of IL-12 and IL-18 on the function of these cells in vitro and in vivo. We observed that in the presence of IL-3, IL-18 causes basophils to release large amounts of IL-4 and IL-13 in vitro. IL-12 modestly enhances this action. We also observed that injection of IL-18 increases the capacity of basophils to produce IL-4, IL-13, and histamine in helminth-infected mice.
Materials and Methods
Mice.
Specific pathogen-free female BALB/c normal or IFN-γ deficient (IFN-γ−/−) mice, 6 weeks of age, were obtained from Japan SLC (Hamamatsu, Japan) or kindly provided by Yoichiro Iwakura (Institute of Medical Science, University of Tokyo, Tokyo), respectively. Generation of IL-18Rα-deficient (IL-18Rα−/−) mice was described in our previous report (25).
Staining and Isolation of FcɛR+ Cells.
Bone marrow cells cultured with IL-3 (20 units/ml) for 10 days in RPMI 1640 supplemented with 10% FBS, 50 μM 2-ME, 2 mM l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin were washed twice. Cells first were incubated with 10 μg/ml anti-FcγRII/III for 30 min at 4°C, followed by 10 μg/ml mouse IgE anti-DNP for 1 h at 4°C in staining buffer (PBS/1% FCS). Cells then were washed twice and stained with FITC-anti-IgE (Southern Biotechnology Associates) and PE-anti-c-kit (PharMingen) for 30 min. Control samples were stained with anti-FcγRII/III and FITC-anti-IgE. Samples were analyzed on a FACS Caliber (Becton Dickinson) and separated into FcɛR+/c-kit− and FcɛR+/c-kit+ cells by fluorescence cell sorting (Elite; Coulter).
In Vivo Treatment of Mice.
BALB/c normal or IFN-γ−/− mice were either not treated or treated with s.c. inoculation of 700 Nippostrongylus brasiliensis (Nb) third-stage larvae on the first day of the experiment. Nb-inoculated wild-type and IFN-γ−/− mice received daily injections of PBS, IL-18 (1 μg/mouse), or IL-12 (50 ng/mouse) and IL-18 (1 μg/mouse) for 7 days. At 8 days after infection, these mice were sacrificed.
Generation and Measurement of Lymphokines and Histamine.
Bone marrow cells cultured with 20 units/ml IL-3 for 10 days were washed and recultured with medium alone or IL-3 (20 units/ml) and/or IL-18 (20 ng/ml) in 96-well plates for 24 h at 2 × 105/0.2 ml per well. For FcɛR-dependent activation, cultured bone marrow cells were sensitized with a mouse IgE anti-DNP for 1 h, washed twice, and restimulated with immobilized anti-IgE in the presence or absence of IL-3 and/or IL-18 for 5 h at 2 × 105/0.2 ml per well. Supernatants were measured for IL-4/IL-13 (R & D Systems) or histamine content (ICN) by ELISA.
Intracellular Cytokine Staining.
For analysis of IL-4-producing cells, we followed the modified protocol of immunofluorescent staining of intracellular cytokines for the flow cytometric analysis described in our previous report (15). Briefly, bone marrow cells cultured with 20 units/ml IL-3 for 10 days were washed and recultured at 2 × 106/ml with IL-3 (20 units/ml) or IL-3 plus IL-18 (20 ng/ml) for 16 h; 3 μg/ml monensin was added for the final 6 h to inhibit IL-4 secretion. Such treated cells were first stained with IgE anti-DNP/FITC-anti-IgE and a combination of biotin-anti-c-kit and Tri-Color-conjugated avidin (Caltag Laboratories, South San Francisco, CA) followed by fixation with 4% (wt/vol) paraformaldehyde in PBS and permeabilization of cell membrane with ice-cold PBS containing 1% FCS plus 0.1% saponin. Resultant cells were stained further with 0.5 μg of PE-rat anti-mouse IL-4 antibody or PE-rat IgG1 isotype-matched antibody (PharMingen). The frequency of FcɛR+/c-kit− and FcɛR+/c-kit+ cells producing IL-4 was determined by gating on the respective cell populations.
Preparation of Splenic Non-B, Non-T Cells and CD4+ T Cells.
For preparation of splenic non-B, non-T cells, spleen cells (2 × 107/ml) were treated with 10 μg/ml FITC-anti-B220 and FITC-anti-CD3 for 60 min at 4°C on a turning wheel. The cells then were washed twice and resuspended with magnetic beads coated with sheep anti-FITC antibodies (PerSeptive Diagnostics, Cambridge, MA). Cells that had bound magnetic beads were depleted by three rounds of exposure to a magnetic field. The residual cells were collected and washed twice, yielding <1% CD3- and B220-positive cells. Splenic CD4+ T cells were purified by MicroBeads (Miltenyi Biotec, Auburn, CA). This procedure routinely yields cells that are >98% CD4+ T cells.
Results
IL-3 and IL-18 Stimulate Cultured Bone Marrow Cells to Produce IL-4 and IL-13 in Vitro.
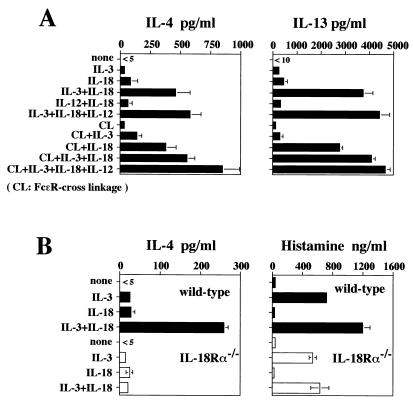
We first examined IL-18 responsiveness of bone marrow cells cultured in IL-3 for 10 days. These cells were stimulated with medium alone or with IL-3 (20 units/ml) and/or IL-18 (20 ng/ml) for 24 h. Cultured bone marrow cells produced modest amounts of IL-4 and IL-13 in response to IL-3 or IL-18 alone but considerable amounts in response to IL-3 plus IL-18 (Fig. 1A). Production of IL-4 and IL-13 was enhanced modestly by additional stimulation with IL-12 (Fig. 1A); however, IL-18 plus IL-12, without IL-3, was ineffective in inducing IL-4 and IL-13 production. These cultured bone marrow cells did not produce IFN-γ in response to any combination of IL-3, IL-18, and IL-12 (data not shown).
Figure 1.
IL-4 and IL-13 production from cultured bone marrow cells in response to IL-18. Bone marrow cells from BALB/c (A) or IL-18Rα-deficient (B) mice cultured with IL-3 (20 units/ml) for 10 days were washed and restimulated at 2 × 105/ml with medium alone or various combinations of IL-3 (20 units/ml), IL-18 (20 ng/ml), and IL-12 (20 ng/ml) with or without FcɛR cross-linking (CL) for 5 or 24 h, respectively. Supernatants were harvested and tested for production of IL-4, IL-13, and histamine by ELISA. Results are geometric means ± SEM.
To attain responsiveness to IL-18, bone marrow cells required IL-3 for at least 7 days; freshly prepared bone marrow cells produced little or no IL-4 and/or IL-13 in response to IL-3 and IL-18 (data not shown). Importantly, IL-3 and IL-18 induce production of IL-4 and IL-13 by cultured bone marrow cells without FcɛR cross-linkage. FcɛR cross-linkage only modestly enhanced cytokine production in response to IL-3 and IL-18 (Fig. 1A). As shown in Fig. 1B, IL-3 can induce substantial histamine release from bone marrow cells, whereas IL-18 only modestly enhances this IL-3-induced release. Furthermore, bone marrow cells derived from wild-type and IL-18Rα−/− mice (25) show similar levels of histamine release in response to IL-3. In contrast, IL-3 and IL-18 only stimulate IL-4 production from wild-type mice, indicating important differences in IL-3 stimulation of histamine release and cytokine production.
IL-3 and IL-18 Stimulate Basophils to Produce IL-4 and IL-13 in Vitro.
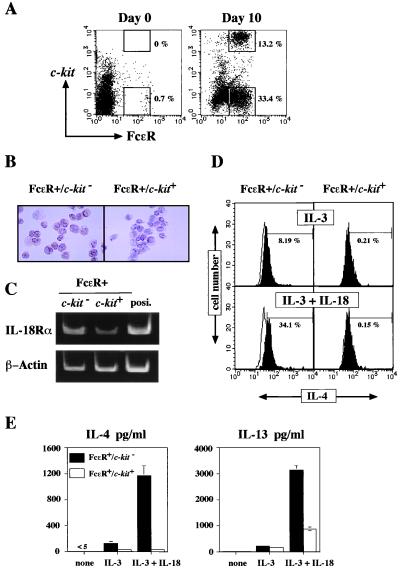
Progenitors in bone marrow develop into mature basophils and mast cells in cultures containing IL-3. As shown in Fig. 2A, a marked increase in the proportion of FcɛR+ cells was observed after a 10-day culture in the presence of IL-3. As reported previously, FcɛR+ cells can be separated into two major populations: FcɛR+/c-kit− and FcɛR+/c-kit+ cells (16, 26–28). Light and electron microscopic examination of the FcɛR+/c-kit+ cells revealed that they were virtually pure mast cells. FcɛR+/c-kit− cells were composed mainly of basophils (26). Thus, c-kit expression can be used to distinguish mast cells from basophils.
Figure 2.
IL-4 and IL-13 production from FcɛR+ cells in response to IL-18. (A) Freshly prepared or 10-day-IL-3-cultured bone marrow cells were analyzed for expression of FcɛR and c-kit by flow cytometry. (B) Bone marrow cells from BALB/c mice cultured with IL-3 for 10 days were sorted into FcɛR+/c-kit− or FcɛR+/c-kit+ cell populations. Surface expression of IL-18Rα was determined immunohistochemically, as described previously (13) (×250). (C) mRNAs extracted from sorted bone marrow cells that had been cultured with IL-3 for 10 days were analyzed for IL-18Rα and β-actin expression by reverse transcription–PCR as described previously (6). As positive controls (posi) for IL-18Rα mRNA, mRNA extracted from murine T cells cultured with IL-12 for 3 days was used (6). (D) Bone marrow cells from BALB/c mice cultured with IL-3 for 10 days were washed and restimulated at 2 × 106/ml with IL-3 (20 units/ml) or IL-3 plus IL-18 (20 ng/ml) for 16 h. Intracellular IL-4 staining was performed and analyzed as described in Materials and Methods. The histograms illustrate the intracellular staining with PE-rat anti-mouse IL-4 antibody (shaded) or PE-rat IgG1 isotype-matched antibody (unshaded, bold line). The percentage shown represents the proportion of IL-4-positive cells. (E) The sorted populations described in B were restimulated with medium alone or IL-3 (20 units/ml) and/or IL-18 (20 ng/ml) for 24 h. Supernatants were analyzed for production of IL-4 and IL-13 by ELISA. Results are geometric means + SEM.
Both basophils and mast cells have been reported to produce IL-4 and IL-13 when stimulated by cross-linkage of FcɛR or with ionomycin (16–24). Therefore, we wished to determine which population, FcɛR+/c-kit− cells (basophils) or FcɛR+/c-kit+ cells (mast cells), was mainly responsible for IL-4 and/or IL-13 production in response to IL-18. To determine whether these FcɛR+ cells express IL-18Rα, basophils and mast cells, purified from IL-3-stimulated bone marrow cells by sorting, were stained immunohistochemically with monoclonal anti-mouse IL-18Rα Ab (13). As shown in Fig. 2B, the majority of FcɛR+/c-kit− cells were strongly positive for IL-18Rα, whereas FcɛR+/c-kit+ cells were weakly positive for IL-18Rα. The specificity of this IL-18Rα staining was verified by the fact that this antibody failed to stain FcɛR+ cells from IL-18Rα−/− mice (data not shown). PCR analysis demonstrated the presence of more IL-18Rα mRNA in basophils than in mast cells (Fig. 2C). These results provide strong evidence that IL-18 can act directly on basophils and mast cells.
To examine the proportion of IL-4-producing cells, we next stained cultured bone marrow cells for cytoplasmic IL-4 and analyzed them by fluorescence-activated cell sorter. Cultured bone marrow cells stimulated with IL-3 or IL-3 plus IL-18 for 16 h were pulsed with 3 μg/ml monensin during the final 6 h to inhibit IL-4 secretion. As shown in Fig. 2D, a substantial proportion (34%) of FcɛR+/c-kit− cells cultured with IL-3 and IL-18 are cytoplasmic IL-4-positive, whereas only a small fraction of FcɛR+/c-kit+ cells are positive for IL-4. Immunohistochemical study provided similar results (data not shown). Next, we purified basophils and mast cells by sorting them from cultured bone marrow cells. As shown in Fig. 2E, basophils produced a large amount of IL-4 and IL-13 in response to IL-3 and IL-18. Although mast cells are poor producers of IL-4, they produced a significant amount of IL-13 in response to IL-3 and IL-18. Thus, among the FcɛR+ cell populations, basophils are the major producers of IL-4 and IL-13 when stimulated with IL-3 and IL-18, whereas mast cells can make some IL-13.
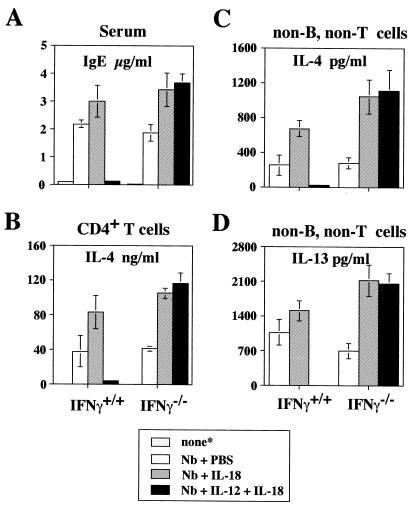
IL-18 Enhances Th2 Responses and the Capacity of Non-B, Non-T Cells in Helminth-Infected Mice to Produce IL-4 and IL-13.
We have shown previously that injection of IL-12 and IL-18 into mice infected with the helminth Nb or injected with anti-IgD antibodies inhibited IgE production by inducing IFN-γ-secreting cells (15). By contrast, the same treatment of IFN-γ−/− mice caused a 2- to 3-fold increase in IgE in response to anti-IgD (15). As shown in Fig. 3A, daily i.p. injection of IL-12 and IL-18 into Nb-infected wild-type mice almost completely inhibited IgE production, whereas such injection increased IgE levels in Nb-infected IFN-γ−/− mice. Furthermore, injection of IL-18 alone increased IgE levels in both wild-type and IFN-γ−/− mice. The development of CD4+ T cells that produce IL-4 and IL-13 (data not shown) in response to plate bound anti-CD3 was completely inhibited by injection of IL-12 and IL-18 into Nb-infected wild-type mice (Fig. 3B). By contrast, CD4+ T cells from IL-18-injected, Nb-infected wild-type mice or from Nb-infected IFN-γ−/− mice that had been treated with IL-18 or IL-12 plus IL-18 increased their production of IL-4 and IL-13 (data not shown) in response to plate-bound anti-CD3 (Fig. 3B).
Figure 3.
IL-18 treatment of Nb-inoculated mice increases IL-4 and IL-13 production by splenic non-B, non-T cells. Wild-type and IFN-γ−/− BALB/c mice either were not treated (*) or infected by s.c. inoculation of 700 Nb third-stage larvae on the first day of the experiment. Nb-inoculated wild-type and IFN-γ−/− BALB/c mice received daily injections of PBS, IL-18 (1 μg/mouse), or IL-12 (50 ng/mouse) and IL-18 (1 μg/mouse) for 7 days. At 8 days after infection, these mice were sacrificed. (A) Serum IgE levels were measured at 8 days after Nb inoculation by ELISA. (B) Splenic CD4+ T cells (2 × 105/0.2 ml per well), purified by MicroBeads (Miltenyi Biotec), from each group of mice were cultured with immobilized anti-CD3 (10 μg/ml for coating) for 24 h. (C and D) Splenic non-B, non-T cells (2 × 105/0.2 ml per well) prepared as described in Materials and Methods were stimulated with FcɛR cross-linking for 24 h. Supernatants were harvested and tested for production of IL-4 and IL-13 by ELISA. Results are geometric means ± SEM.
It has been reported that infection with Nb results in an increase in the number of splenic FcɛR+ non-B, non-T cells (29). Most of these FcɛR+ cells were basophils morphologically and produced IL-4 in response to FcɛR cross-linkage, suggesting that basophil-derived IL-4 may play a physiologically important role in IgE production (21, 22). Therefore, we next examined the effect of injection of IL-18 with or without IL-12 on the capacity of non-B, non-T cells from Nb-infected wild-type and IFN-γ−/− mice to produce IL-4 and IL-13 (Fig. 3 C and D). Splenic FcɛR+ non-B, non-T cells from Nb-inoculated wild-type mice produced significant amounts of IL-4 and IL-13 in response to FcɛR cross-linkage, whereas those from IL-12- and IL-18-injected Nb-inoculated mice produced little. By contrast, such treatment strikingly enhanced IL-4 and IL-13 production by non-B, non-T cells from Nb-inoculated IFN-γ−/− mice. Furthermore, IL-18 without IL-12 enhanced IL-4 and IL-13 production from Nb-infected wild-type mice and IFN-γ−/− mice. Thus, IL-18 without IL-12 stimulates basophils but with IL-12 abrogates their capacity to produce IL-4 and IL-13 in response to FcɛR cross-linkage. The latter is IFN-γ-dependent.
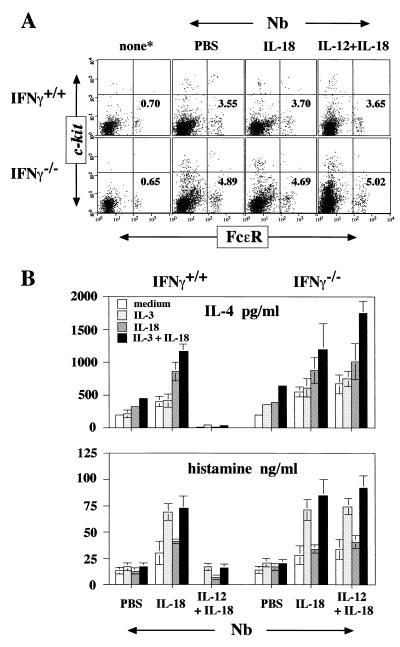
Finally, we examined the capacity of non-B, non-T cells from each group of mice to produce IL-4 and histamine in response to IL-3 and/or IL-18 in vitro. Although the proportion of FcɛR+/c-kit− cells (basophils) was increased strikingly after infection with Nb, treatment of Nb-infected wild-type or IFN-γ−/− mice with IL-18 or with IL-12 and IL-18 did not change this proportion (Fig. 4A). As shown in Fig. 4B, splenic non-B, non-T cells from Nb-inoculated wild-type mice spontaneously produced IL-4 and histamine in vitro and further increased IL-4 production in response to IL-3 and IL-18. Those from IL-12- and IL-18-treated Nb-infected mice did not show this property, although they produced histamine in response to IL-3. Non-B, non-T cells derived from Nb-infected IFN-γ−/− mice treated with IL-12 and IL-18 showed marked enhancement in IL-4 production and histamine release in response to IL-3 plus IL-18. Similarly, non-B, non-T cells from IL-18-treated, Nb-infected wild-type or IFN-γ−/− mice produced high levels of IL-4 and histamine in response to IL-3 and IL-18. These results taken together indicate that in the absence of IFN-γ, IL-18 with or without IL-12 prepares and stimulates basophils to produce IL-4 and IL-13 in vivo as well as in vitro. They further suggest that IFN-γ strikingly diminishes IL-4 and IL-13 production by basophils stimulated with cytokines or by FcɛR cross-linkage.
Figure 4.
Production of IL-4 and histamine by splenic non-B, non-T cells from Nb-inoculated mice. (A) Freshly prepared splenic non-B, non-T cells from each group of mice described in Fig. 3 were analyzed for expression of FcɛR and c-kit by flow cytometry. The percentages shown represent the proportion of FcɛR+/c-kit− cells. (B) Splenic non-B, non-T cells (2 × 105/0.2 ml per well) from each group of mice were stimulated with medium alone or IL-3 and/or IL-18 without FcɛR cross-linking for 24 h. Supernatants were analyzed for production of IL-4 and histamine. Results are geometric means ± SEM.
Discussion
IL-18 has been regarded principally as a cytokine that strongly biases immune responses toward IFN-γ production and, thus, toward effective cellular immunity (1–3, 6, 15). In apparent confirmation of that role, its use with IL-12 strikingly inhibited IgE production in normal mice infected with Nb and markedly diminished the capacity of splenic basophils from these mice to produce IL-4 and IL-13, major cytokine inducers and effectors of allergic inflammation (Figs. 3 and 4). These results can be ascribed to IL-18’s potent stimulation of IFN-γ production by NK cells (30) and by the induction of Th1 cells and the stimulation of their production of IFN-γ through the combined action of IL-12 and IL-18 (4–6). This activity obscures a striking and unanticipated effect of IL-18: its role as a potent costimulant of basophil IL-4 and IL-13 production. Not only does IL-18 strikingly enhance both IL-3- and FcɛR cross-linkage-induced IL-4 and IL-13 production by basophils in vitro (Figs. 1 and 2), IL-18 and IL-12 treatment of Nb-infected mice that cannot produce IFN-γ strikingly enhances IL-4 and IL-13 production by their basophils in response to either FcɛR cross-linkage or to cytokines (Figs. 3 and 4). Furthermore, the increase in IL-4 and IL-13 production by CD4+ T cells in response to anti-CD3 suggests that such treatment of Nb-infected IFN-γ−/− mice has induced Th2 polarization of CD4+ cells (Fig. 3). IL-18 emerges, paradoxically, as a powerful inducer of basophil cytokine production and, thus, potentially, of allergic inflammatory diseases. Thus, although administration of IL-18 and IL-12, or of drugs that express agonist function for these cytokines, could be considered as a treatment for severe allergic disorders, our results inject a major note of caution. In circumstances in which IFN-γ production may be deficient, treatment with IL-18 could markedly enhance allergic responses. Indeed, if IFN-γ is inhibited, IL-18 may have the potential to act as a Th2 polarizer, perhaps through its stimulation of basophil IL-4 production (Fig. 3). Basophil-derived IL-4 potentially could polarize antigen-stimulated CD4+ T cells toward a Th2 phenotype and could account for the enhanced IgE production observed when IL-18 and IL-12 are administered to IFN-γ−/− mice treated with anti-IgD (15). Another possibility is that basophil-derived IL-4 itself, possibly in collaboration with CD40 ligand expressed on CD4+ T cells and/or basophils, may allow stimulated B cells to switch to IgE production. Indeed, it has been reported previously that basophils can play a role in Ig class switching to IgE (31).
A particularly striking finding is the distinct behavior of basophils that develop in Nb-infected mice treated with IL-12 and IL-18. In wild-type mice, where this treatment leads to IFN-γ production, these basophils produce little or no IL-4 in response to both receptor cross-linkage and cytokine stimulation. However, in the absence of IFN-γ, IL-12 and IL-18 treatment actually enhances their IL-4/IL-13-producing activity (Fig. 4). This suggests that differentiation of basophils in the presence or absence of IFN-γ in one aspect or of IL-18 and/or IL-12 in another aspect powerfully determines their cytokine-producing potentiality. Indeed, the proportion of basophils in bone marrow cells cultured with IL-3 plus IFN-γ for 10 days is the same as that in IL-3-cultured bone marrow. However, if these basophils are pretreated with IFN-γ, their IL-4 production is diminished significantly in response to either FcɛR cross-linkage or to cytokines (T.Y., H.F., and K.N., unpublished work). Thus, cytokine agonists or antagonists potentially could determine the differentiated function of developing basophils and may allow alternative strategies for the treatment of allergic diseases.
Acknowledgments
We express sincere thanks to Hayashibara Biochemical Laboratories for providing us with recombinant murine IL-12, IL-18, and monoclonal anti-IL-18Rα antibody. We are grateful to Ms. Hisae Fukui for excellent technical assistance. This study is supported by a Grant-in-Aid for Scientific Research and Hitech Research Center grant from the Ministry of Education, Science, and Culture of Japan.
Abbreviations
- Th1
T helper 1
- FcɛR1
high-affinity IgE receptor
- IFN-γ−/−
IFN-γ-deficient IL-18Rα−/−, IL-18Rα-deficient
- NK
natural killer
- Nb
Nippostrongylus brasiliensis
References
- 1.Okamura H, Tsutsui H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, et al. Nature (London) 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 2.Ushio S, Namba M, Okura T, Hattori K, Nukada Y, Akita K, Tanabe F, Konishi K, Micallef M, Fujii M, et al. J Immunol. 1996;156:4274–4279. [PubMed] [Google Scholar]
- 3.Okamura H, Tsutsui H, Kashiwamura S-I, Yoshimoto T, Nakanishi K. Adv Immunol. 1998;70:281–312. doi: 10.1016/s0065-2776(08)60389-2. [DOI] [PubMed] [Google Scholar]
- 4.Kohno K, Kataoka J, Ohtsuki T, Suemoto Y, Okamoto I, Usui M, Ikeda M, Kurimoto M. J Immunol. 1997;158:1541–1550. [PubMed] [Google Scholar]
- 5.Robinson D, Shibuya K, Mui A, Zonin F, Murphy E, Sana T, Hartley S B, Menon S, Kastelein R, Bazan F, et al. Immunity. 1997;7:571–581. doi: 10.1016/s1074-7613(00)80378-7. [DOI] [PubMed] [Google Scholar]
- 6.Yoshimoto T, Takeda K, Tanaka T, Ohkusu K, Kasiwamura S-I, Okamura H, Akira S, Nakanishi K. J Immunol. 1998;161:3400–3407. [PubMed] [Google Scholar]
- 7.Dao T, Ohashi K, Kayano T. Cell Immunol. 1996;173:230–235. doi: 10.1006/cimm.1996.0272. [DOI] [PubMed] [Google Scholar]
- 8.Tsutsui H, Nakanishi K, Matsui K, Higashino K, Okamura H, Miyazawa Y, Kaneda K. J Immunol. 1996;157:3967–3973. [PubMed] [Google Scholar]
- 9.Torigoe K, Ushio S, Okura T, Kobayashi S, Taniai M, Kunikata T, Murakami T, Sanou O, Kojima H, Fuji M, et al. J Biol Chem. 1997;272:25737–25742. doi: 10.1074/jbc.272.41.25737. [DOI] [PubMed] [Google Scholar]
- 10.Born T L, Thomassen E, Bird T A, Sims J E. J Biol Chem. 1998;273:29445–29450. doi: 10.1074/jbc.273.45.29445. [DOI] [PubMed] [Google Scholar]
- 11.Dinarello C A. J Allergy Clin Immunol. 1999;103:11–24. doi: 10.1016/s0091-6749(99)70518-x. [DOI] [PubMed] [Google Scholar]
- 12.Xu D, Chan W L, Leung B P, Hunter D, Schulz K, Carter R W, McInnes I B, Robinson J H, Liew F Y. J Exp Med. 1998;188:1485–1492. doi: 10.1084/jem.188.8.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hyodo Y, Matsui K, Hayashi N, Tsutsui H, Kashiwamura S-I, Yamauchi H, Hiroishi K, Takeda K, Tagawa Y-I, Iwakura Y, et al. J Immunol. 1999;162:1662–1668. [PubMed] [Google Scholar]
- 14.Hoshino T, Wiltrout R H, Young H A. J Immunol. 1999;162:5070–5077. [PubMed] [Google Scholar]
- 15.Yoshimoto T, Okamura H, Tagawa Y-I, Iwakura Y, Nakanishi K. Proc Natl Acad Sci USA. 1997;94:3948–3953. doi: 10.1073/pnas.94.8.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paul W E, Seder R A, Plaut M. Adv Immunol. 1993;53:1–29. [PubMed] [Google Scholar]
- 17.Costa J J, Weller P F, Galli S J. J Am Med Assoc. 1997;278:1815–1822. [PubMed] [Google Scholar]
- 18.Galli S J, Lantz C S. In: Fundamental Immunology. 4th Ed. Paul W E, editor. Philadelphia: Lippincott; 1999. pp. 1137–1184. [Google Scholar]
- 19.Plaut M, Pierce J H, Watson C J, Hanley-Hyde J, Nordan R P, Paul W E. Nature (London) 1989;339:64–67. doi: 10.1038/339064a0. [DOI] [PubMed] [Google Scholar]
- 20.Burd P R, Rogers H W, Gordon J R, Martin C A, Jayaraman S, Wilson S D, Dvorak A M, Galli S J, Dorf M E. J Exp Med. 1989;170:245–257. doi: 10.1084/jem.170.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seder R A, Plaut M, Barbieri S, Urban J, Jr, Finkelman F D, Paul W E. J Immunol. 1991;147:903–909. [PubMed] [Google Scholar]
- 22.Seder R A, Paul W E, Dvorak A M, Sharkis S J, Kagey-Sobotka A, Niv Y, Finkelman F D, Barbieri S A, Galli S J, Plaut M. Proc Natl Acad Sci USA. 1991;88:2835–2839. doi: 10.1073/pnas.88.7.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burd P R, Thompson W C, Max E E, Mills F C. J Exp Med. 1995;181:1373–1380. doi: 10.1084/jem.181.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ochensberger B, Daepp G C, Rihs S, Dahinden C A. Blood. 1996;88:3028–3037. [PubMed] [Google Scholar]
- 25.Hoshino K, Tsutsui H, Kawai T, Takeda K, Nakanishi K, Takeda Y, Akira A. J Immunol. 1999;162:5041–5044. [PubMed] [Google Scholar]
- 26.Dvorak A M, Seder R A, Paul W E, Morgan E S, Galli S J. Am J Pathol. 1994;144:160–170. [PMC free article] [PubMed] [Google Scholar]
- 27.Kinzer C A, Keegan A D, Paul W E. J Exp Med. 1995;182:575–579. doi: 10.1084/jem.182.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dvorak A M. Int Arch Allergy Immunol. 1997;114:1–9. doi: 10.1159/000237635. [DOI] [PubMed] [Google Scholar]
- 29.Conrad D H, Ben-Sasson S Z, Le Gros G, Finkelman F D, Paul W E. J Exp Med. 1990;171:1497–1508. doi: 10.1084/jem.171.5.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsutsui H, Matsui K, Kawada N, Hyodo Y, Hayashi N, Okamura H, Higashino K, Nakanishi K. J Immunol. 1997;159:3961–3967. [PubMed] [Google Scholar]
- 31.Gauchat J F, Henchoz S, Mazzei G, Aubry J P, Brunner T, Blasey H, Life P, Talabot D, Flores-Romo L, Thompson J, et al. Nature (London) 1993;365:340–343. doi: 10.1038/365340a0. [DOI] [PubMed] [Google Scholar]