Abstract
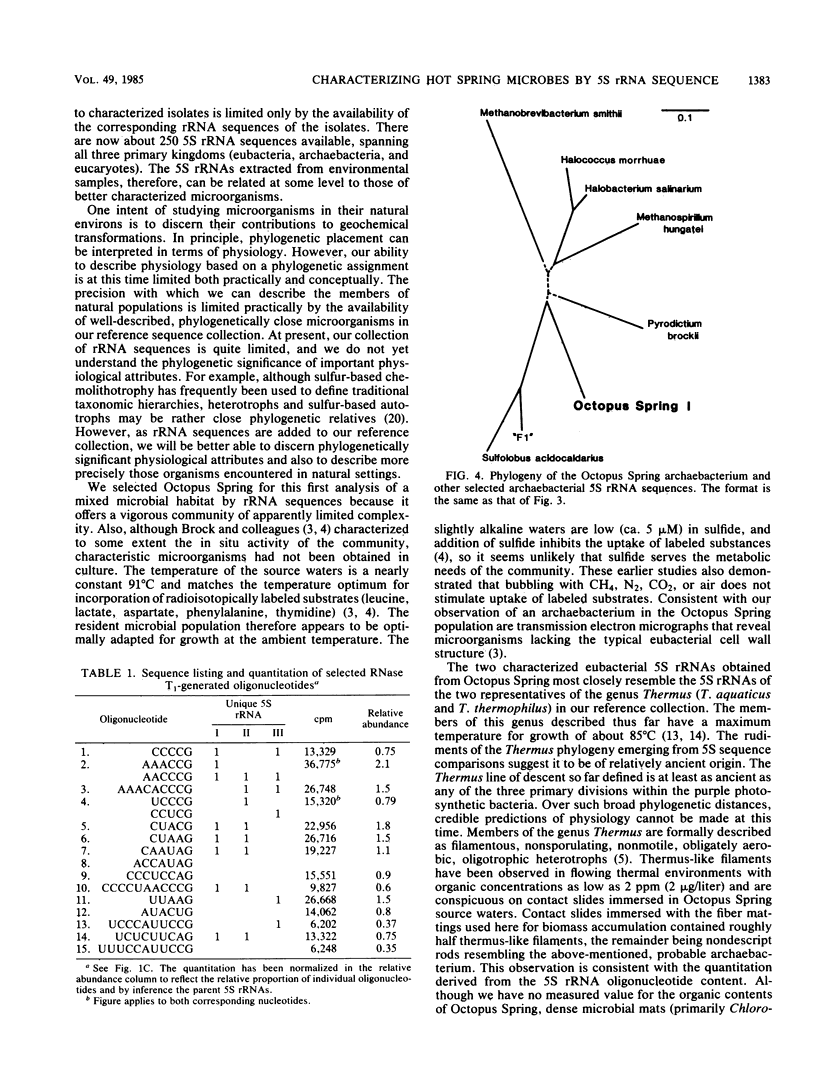
The microorganisms inhabiting a 91 degrees C hot spring in Yellowstone National Park were characterized by sequencing 5S rRNAs isolated from the mixed, natural microflora without cultivation. By comparisons of these sequences with reference sequences, the phylogenetic relationships of the hot spring organisms to better characterized ones were established. Quantitation of the total 5S-sized rRNAs revealed a complex microbial community of three dominant members, a predominant archaebacterium affiliated with the sulfur-metabolizing (dependent) branch of the archaebacteria, and two eubacteria distantly related to Thermus spp. The archaebacterial and the eubacterial 5S rRNAs each constituted about half the examined population.
Full text
PDF
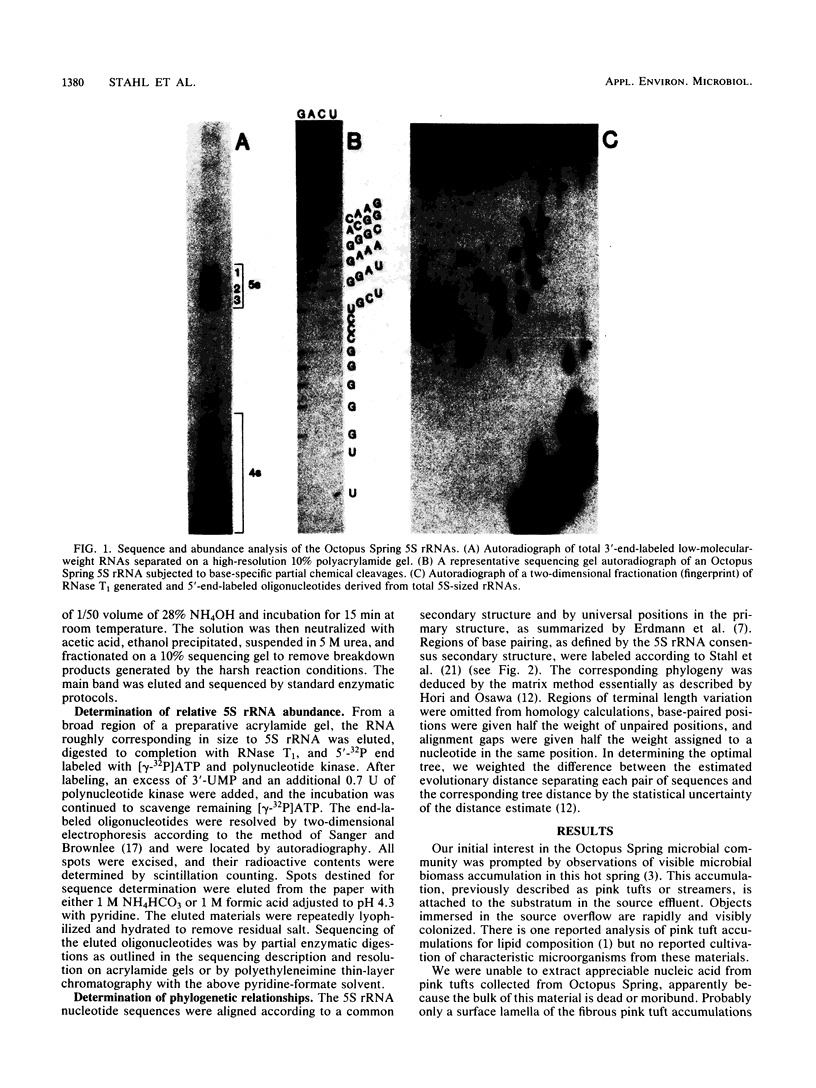
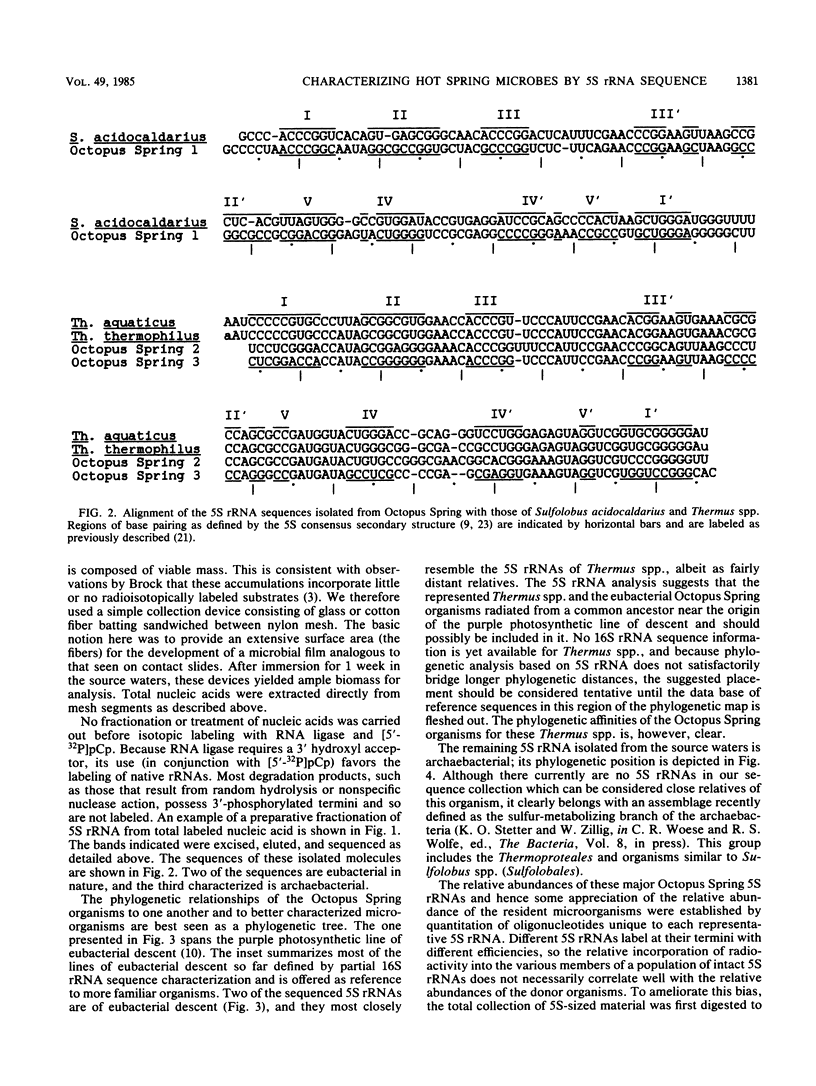
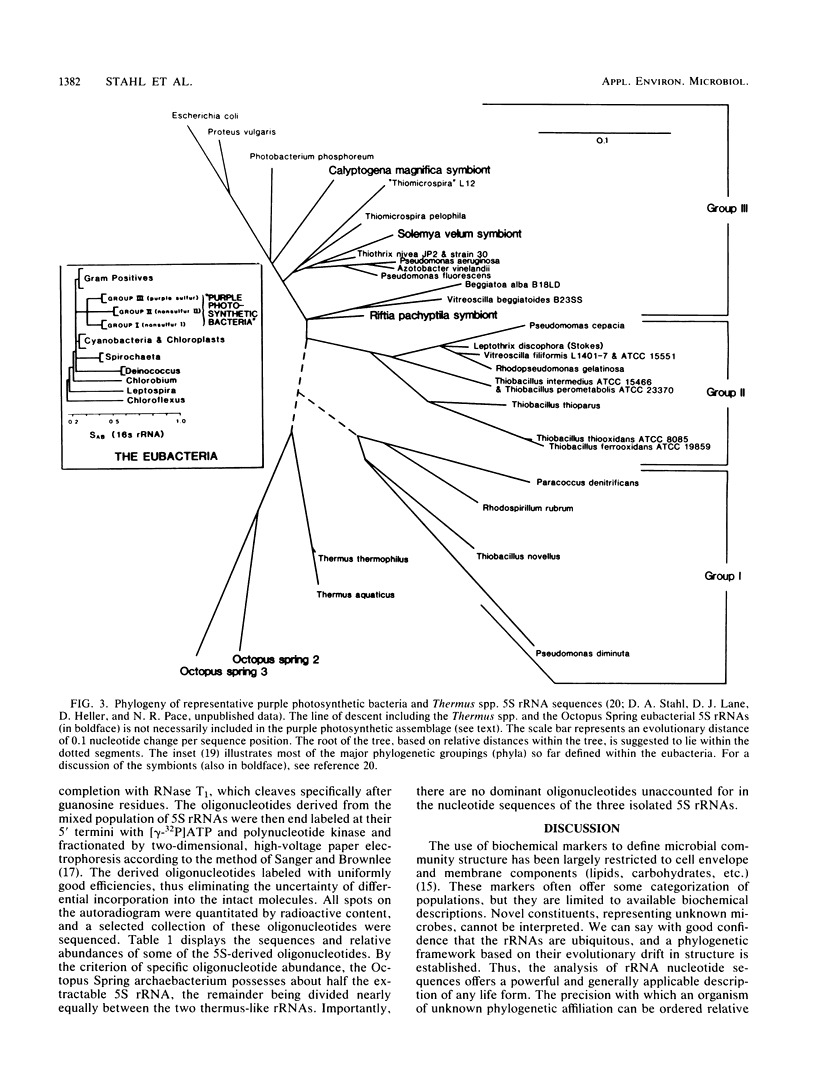

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauman A. J., Simmonds P. G. Fatty acids and polar lipids of extremely thermophilic filamentous bacterial masses from two Yellowstone hot springs. J Bacteriol. 1969 May;98(2):528–531. doi: 10.1128/jb.98.2.528-531.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock T. D., Brock M. L. Temperature optimum of non-sulphur bacteria from a spring at 90C. Nature. 1971 Oct 15;233(5320):494–495. doi: 10.1038/233494a0. [DOI] [PubMed] [Google Scholar]
- Brock T. D., Freeze H. Thermus aquaticus gen. n. and sp. n., a nonsporulating extreme thermophile. J Bacteriol. 1969 Apr;98(1):289–297. doi: 10.1128/jb.98.1.289-297.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock T. D. Life at high temperatures. Evolutionary, ecological, and biochemical significance of organisms living in hot springs is discussed. Science. 1967 Nov;158(3804):1012–1019. doi: 10.1126/science.158.3804.1012. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann V. A., Wolters J., Huysmans E., Vandenberghe A., De Wachter R. Collection of published 5S and 5.8S ribosomal RNA sequences. Nucleic Acids Res. 1984;12 (Suppl):r133–r166. doi: 10.1093/nar/12.suppl.r133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox G. E., Stackebrandt E., Hespell R. B., Gibson J., Maniloff J., Dyer T. A., Wolfe R. S., Balch W. E., Tanner R. S., Magrum L. J. The phylogeny of prokaryotes. Science. 1980 Jul 25;209(4455):457–463. doi: 10.1126/science.6771870. [DOI] [PubMed] [Google Scholar]
- Fox G. E., Woese C. R. 5S RNA secondary structure. Nature. 1975 Aug 7;256(5517):505–507. doi: 10.1038/256505a0. [DOI] [PubMed] [Google Scholar]
- Hayatsu H., Wataya Y., Kai K., Iida S. Reaction of sodium bisulfite with uracil, cytosine, and their derivatives. Biochemistry. 1970 Jul 7;9(14):2858–2865. doi: 10.1021/bi00816a016. [DOI] [PubMed] [Google Scholar]
- Hori H., Osawa S. Evolutionary change in 5S RNA secondary structure and a phylogenic tree of 54 5S RNA species. Proc Natl Acad Sci U S A. 1979 Jan;76(1):381–385. doi: 10.1073/pnas.76.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristjansson J. K., Alfredsson G. A. Distribution of Thermus spp. in Icelandic Hot Springs and a Thermal Gradient. Appl Environ Microbiol. 1983 Jun;45(6):1785–1789. doi: 10.1128/aem.45.6.1785-1789.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J. H., Smith G. A., Fredrickson H. L., Vestal J. R., White D. C. Sensitive assay, based on hydroxy fatty acids from lipopolysaccharide lipid A, for Gram-negative bacteria in sediments. Appl Environ Microbiol. 1982 Nov;44(5):1170–1177. doi: 10.1128/aem.44.5.1170-1177.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setchell W. A. THE UPPER TEMPERATURE LIMITS OF LIFE. Science. 1903 Jun 12;17(441):934–937. doi: 10.1126/science.17.441.934. [DOI] [PubMed] [Google Scholar]
- Stahl D. A., Lane D. J., Olsen G. J., Pace N. R. Analysis of hydrothermal vent-associated symbionts by ribosomal RNA sequences. Science. 1984 Apr 27;224(4647):409–411. doi: 10.1126/science.224.4647.409. [DOI] [PubMed] [Google Scholar]
- Stahl D. A., Luehrsen K. R., Woese C. R., Pace N. R. An unusual 5S rRNA, from Sulfolobus acidocaldarius, and its implications for a general 5S rRNA structure. Nucleic Acids Res. 1981 Nov 25;9(22):6129–6137. doi: 10.1093/nar/9.22.6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studnicka G. M., Eiserling F. A., Lake J. A. A unique secondary folding pattern for 5S RNA corresponds to the lowest energy homologous secondary structure in 17 different prokaryotes. Nucleic Acids Res. 1981 Apr 24;9(8):1885–1904. doi: 10.1093/nar/9.8.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerkandl E., Pauling L. Molecules as documents of evolutionary history. J Theor Biol. 1965 Mar;8(2):357–366. doi: 10.1016/0022-5193(65)90083-4. [DOI] [PubMed] [Google Scholar]