Abstract
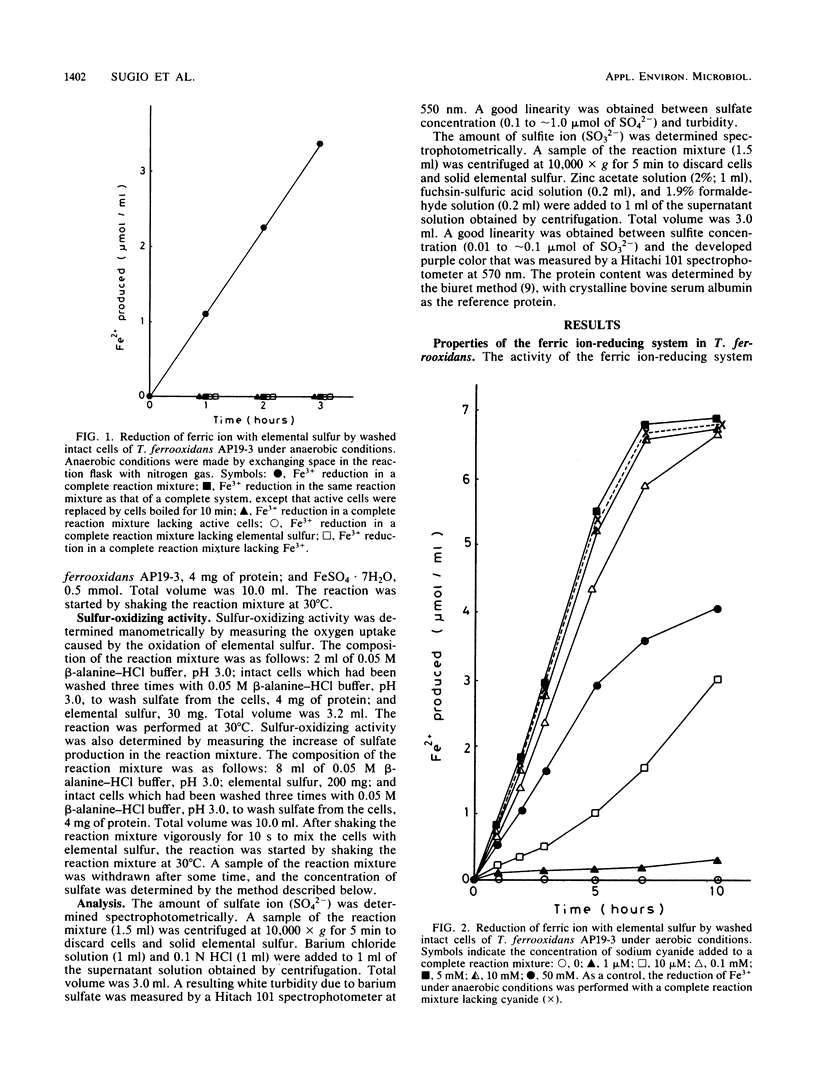
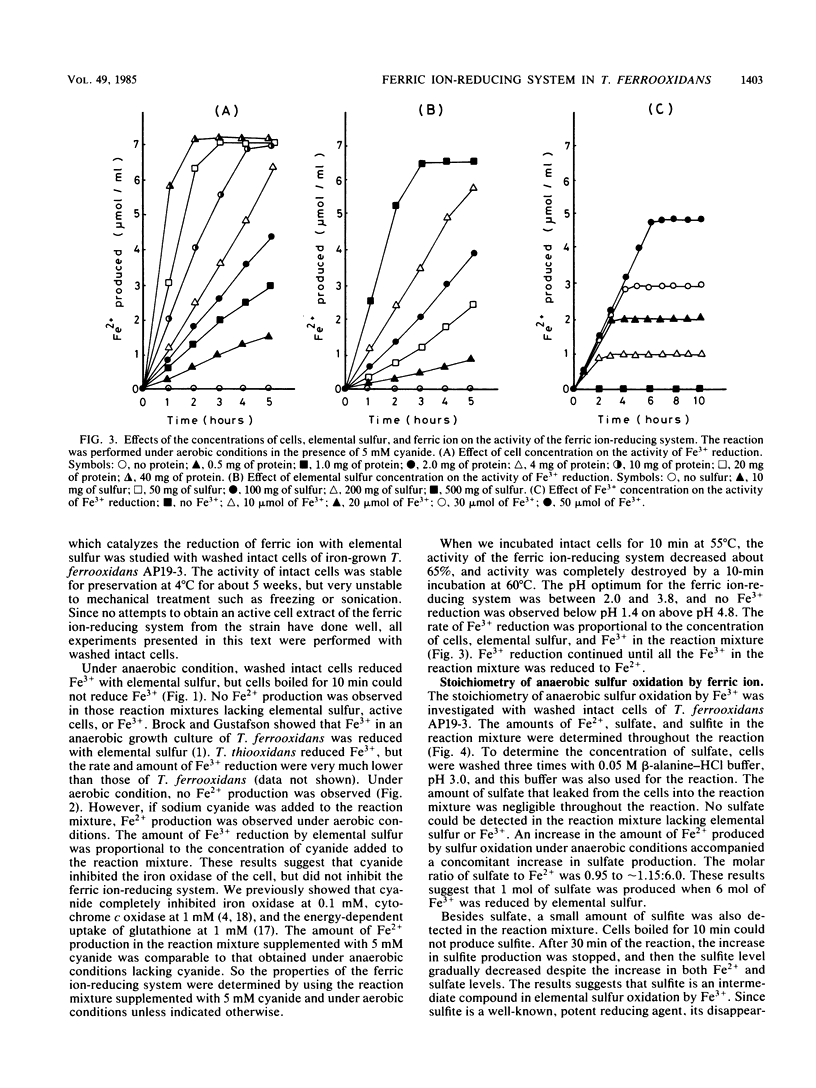
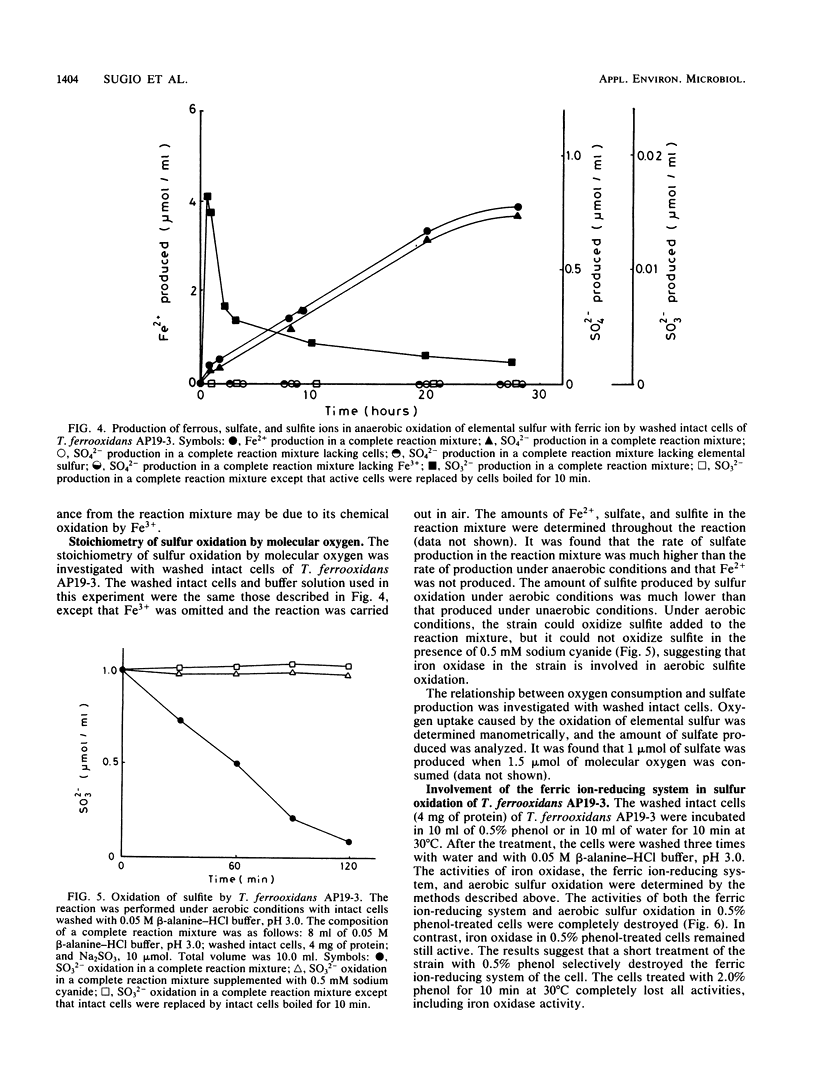
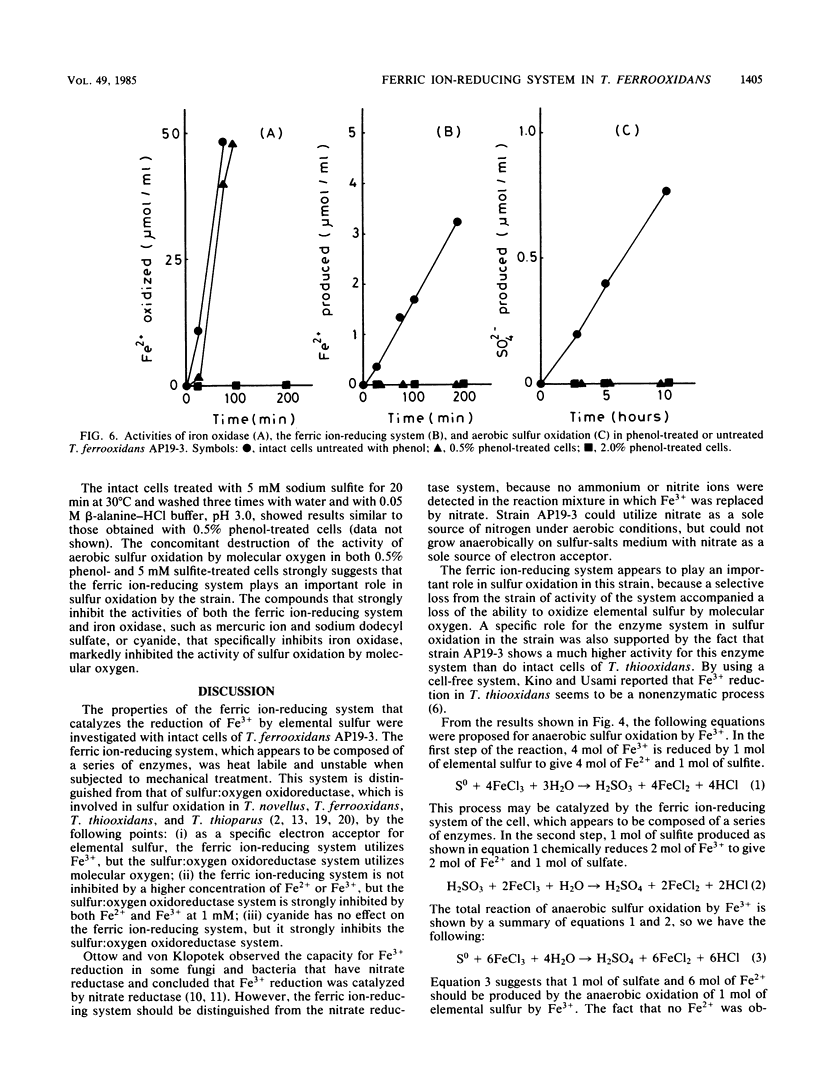
The properties of a ferric ion-reducing system which catalyzes the reduction of ferric ion with elemental sulfur was investigated with a pure strain of Thiobacillus ferrooxidans. In anaerobic conditions, washed intact cells of the strain reduced 6 mol of Fe3+ with 1 mol of elemental sulfur to give 6 mol of Fe2+, 1 mol of sulfate, and a small amount of sulfite. In aerobic conditions, the 6 mol of Fe2+ produced was immediately reoxidized by the iron oxidase of the cell, with a consumption of 1.5 mol of oxygen. As a result, Fe2+ production was never observed under aerobic conditions. However, in the presence of 5 mM cyanide, which completely inhibits the iron oxidase of the cell, an amount of Fe2+ production comparable to that formed under anaerobic conditions was observed under aerobic conditions. The ferric ion-reducing system had a pH optimum between 2.0 and 3.8, and the activity was completely destroyed by 10 min of incubation at 60°C. A short treatment of the strain with 0.5% phenol completely destroyed the ferric ion-reducing system of the cell. However, this treatment did not affect the iron oxidase of the cell. Since a concomitant complete loss of the activity of sulfur oxidation by molecular oxygen was observed in 0.5% phenol-treated cells, it was concluded that the ferric ion-reducing system plays an important role in the sulfur oxidation activity of this strain, and a new sulfur-oxidizing route is proposed for T. ferrooxidans.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brock T. D., Gustafson J. Ferric iron reduction by sulfur- and iron-oxidizing bacteria. Appl Environ Microbiol. 1976 Oct;32(4):567–571. doi: 10.1128/aem.32.4.567-571.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccleston M., Kelly D. P. Oxidation kinetics and chemostat growth kinetics of Thiobacillus ferrooxidans on tetrathionate and thiosulfate. J Bacteriol. 1978 Jun;134(3):718–727. doi: 10.1128/jb.134.3.718-727.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KINSEL N. A. New sulfur oxidizing iron bacterium: Ferrobacillus sulfooxidans sp. n. J Bacteriol. 1960 Nov;80:628–632. doi: 10.1128/jb.80.5.628-632.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D. P. Biochemistry of the chemolithotrophic oxidation of inorganic sulphur. Philos Trans R Soc Lond B Biol Sci. 1982 Sep 13;298(1093):499–528. doi: 10.1098/rstb.1982.0094. [DOI] [PubMed] [Google Scholar]
- Landesman J., Duncan D. W., Walden C. C. Oxidation of inorganic sulfur compounds by washed cell suspensions of Thiobacillus ferrooxidans. Can J Microbiol. 1966 Oct;12(5):957–964. doi: 10.1139/m66-129. [DOI] [PubMed] [Google Scholar]
- Ottow J. C. Evaluation of iron-reducing bacteria in soil and the physiological mechanism of iron-reduction in Aerobacter aerogenes. Z Allg Mikrobiol. 1968;8(5):441–443. doi: 10.1002/jobm.3630080512. [DOI] [PubMed] [Google Scholar]
- Ottow J. C., Von Klopotek A. Enzymatic reduction of iron oxide by fungi. Appl Microbiol. 1969 Jul;18(1):41–43. doi: 10.1128/am.18.1.41-43.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILVERMAN M. P., LUNDGREN D. G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. II. Manometric studies. J Bacteriol. 1959 Sep;78:326–331. doi: 10.1128/jb.78.3.326-331.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver M., Lundgren D. G. Sulfur-oxidizing enzyme of Ferrobacillus ferrooxidans (Thiobacillus ferrooxidans). Can J Biochem. 1968 May;46(5):457–461. doi: 10.1139/o68-069. [DOI] [PubMed] [Google Scholar]
- Silver M., Lundgren D. G. The thiosulfate-oxidizing enzyme of Ferrobacillus ferrooxidans (Thiobacillus ferrooxidans). Can J Biochem. 1968 Oct;46(10):1215–1220. doi: 10.1139/o68-181. [DOI] [PubMed] [Google Scholar]
- Silver M. Oxidation of elemental sulfur and sulfur compounds and CO2 fixation by Ferrobacillus ferrooxidans (Thiobacillus ferrooxidans). Can J Microbiol. 1970 Sep;16(9):845–849. doi: 10.1139/m70-142. [DOI] [PubMed] [Google Scholar]
- Sugio T., Domatsu C., Tano T., Imai K. Role of Ferrous Ions in Synthetic Cobaltous Sulfide Leaching of Thiobacillus ferrooxidans. Appl Environ Microbiol. 1984 Sep;48(3):461–467. doi: 10.1128/aem.48.3.461-467.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki I. Oxidation of elemental sulfur by an enzyme system of Thiobacillus thiooxidans. Biochim Biophys Acta. 1965 Jul 8;104(2):359–371. doi: 10.1016/0304-4165(65)90341-7. [DOI] [PubMed] [Google Scholar]
- Suzuki I., Silver M. The initial product and properties of the sulfur-oxidizing enzyme of thiobacilli. Biochim Biophys Acta. 1966 Jul 6;122(1):22–33. doi: 10.1016/0926-6593(66)90088-9. [DOI] [PubMed] [Google Scholar]
- Tuovinen P. H., Kelley B. C., Nicholas D. J. Enzymic comparisons of the inorganic sulfur metabolism in autotrophic and heterotrophic Thiobacillus ferrooxidans. Can J Microbiol. 1976 Jan;22(1):109–113. doi: 10.1139/m76-016. [DOI] [PubMed] [Google Scholar]
- Vestal J. R., Lundgren D. G. The sulfite oxidase of Thiobacillus ferrooxidans (Ferrobacillus ferrooxidans). Can J Biochem. 1971 Oct;49(10):1125–1130. doi: 10.1139/o71-162. [DOI] [PubMed] [Google Scholar]


