Abstract
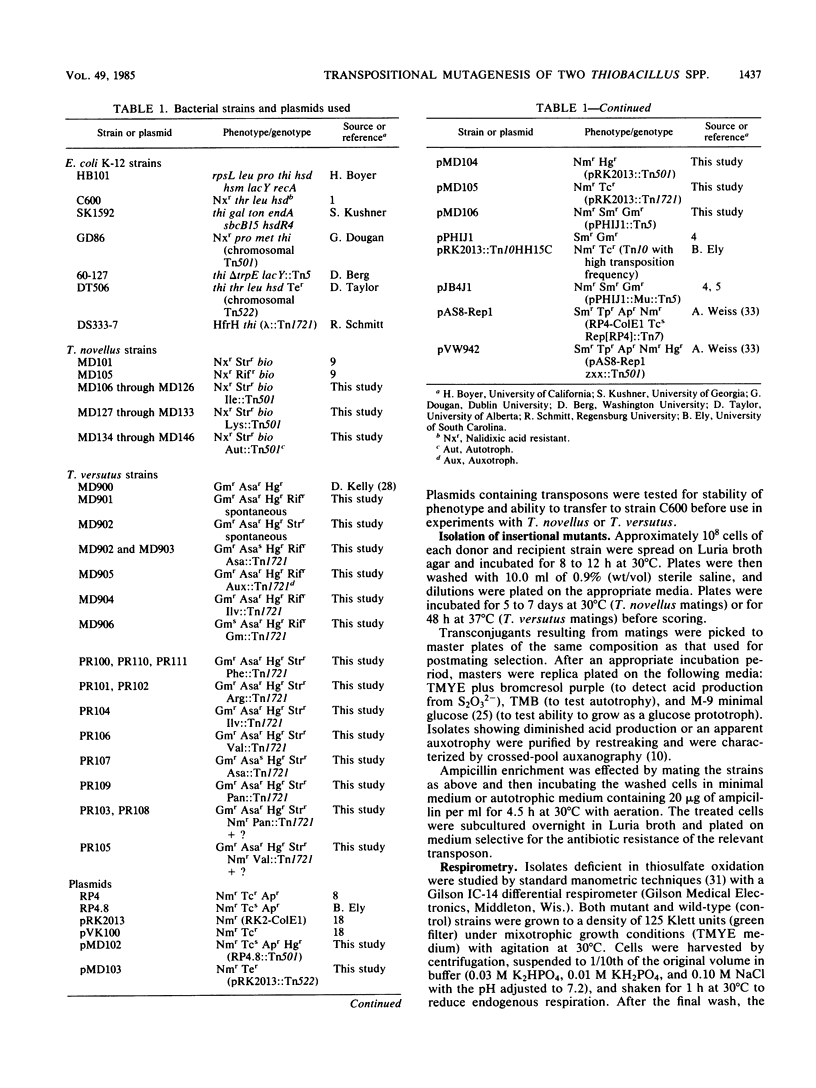
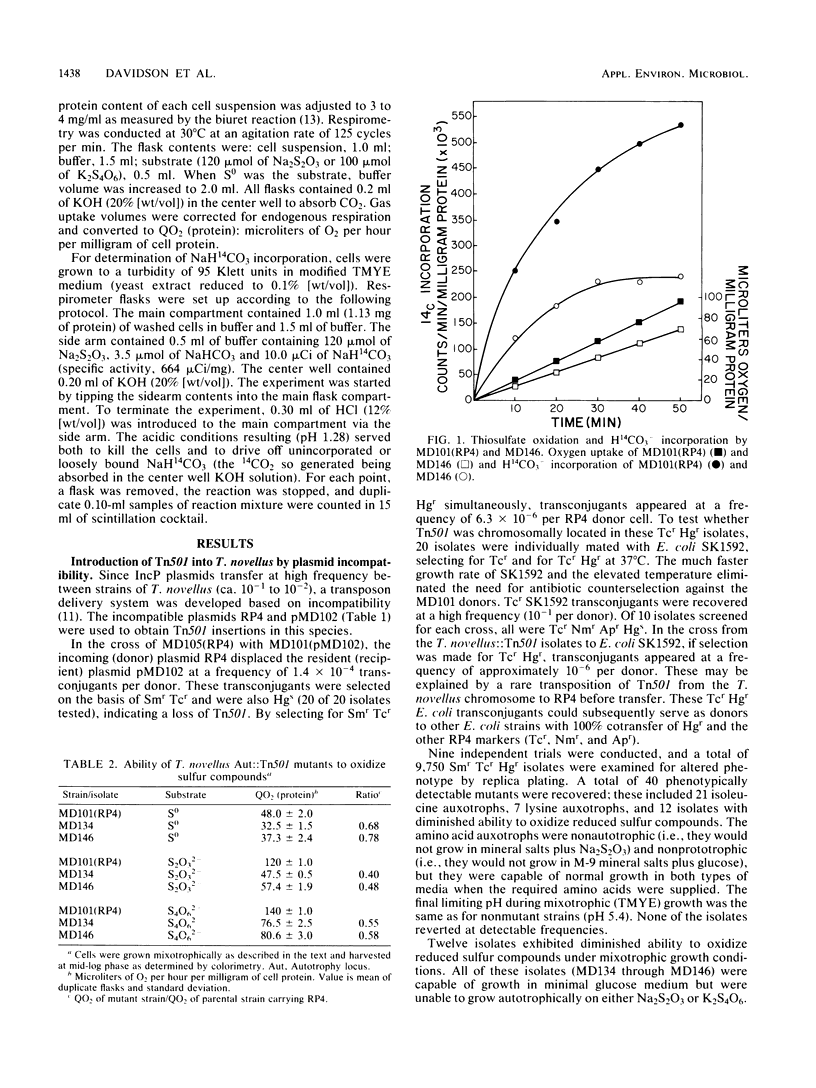
Transpositional mutagenesis of Thiobacillus novellus by Tn501 was achieved by means of the incompatibility of IncP plasmids. Tn501 insertion caused three types of mutant phenotypes: isoleucine auxotrophy, lysine auxotrophy, and a reduced ability to oxidize reduced sulfur compounds and to fix CO2. Oxidation rates for elemental sulfur (S0), thiosulfate (S2O32−), and tetrathionate (S4O62−) in mutants of the latter type were reduced relative to those of the nonmutant control strain. Incorporation of labeled bicarbonate (H14CO3−) was also significantly impaired. Although suicide vehicles were not useful for the introduction of transposons into T. novellus, this method was effective for the Tn1721-induced mutagenesis of Thiobacillus versutus. Tn1721 insertions resulted in the loss of the natural resistance of T. versutus to arsenate and gentamicin and in auxotrophies for isoleucine-valine, arginine, phenylalanine, valine, and panthothenate. Transpositional mutagenesis by either method should prove to be a useful tool for further study of these and other members of the genus Thiobacillus.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles A. M. Effect of growth substrate on enzymes of the citric and glyoxylic acid cycles in Thiobacillus novellus. Can J Microbiol. 1971 May;17(5):617–624. doi: 10.1139/m71-101. [DOI] [PubMed] [Google Scholar]
- Chatterjee A. K., Thurn K. K., Feese D. A. Tn5-Induced Mutations in the Enterobacterial Phytopathogen Erwinia chrysanthemi. Appl Environ Microbiol. 1983 Feb;45(2):644–650. doi: 10.1128/aem.45.2.644-650.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Hedges R. W., Shaw E. J., Sykes R. B., Richmond M. H. Properties of an R factor from Pseudomonas aeruginosa. J Bacteriol. 1971 Dec;108(3):1244–1249. doi: 10.1128/jb.108.3.1244-1249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson M. S., Summers A. O. Wide-host-range plasmids function in the genus thiobacillus. Appl Environ Microbiol. 1983 Sep;46(3):565–572. doi: 10.1128/aem.46.3.565-572.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely B., Croft R. H. Transposon mutagenesis in Caulobacter crescentus. J Bacteriol. 1982 Feb;149(2):620–625. doi: 10.1128/jb.149.2.620-625.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely B. Transfer of drug resistance factors to the dimorphic bacterium Caulobacter crescentus. Genetics. 1979 Mar;91(3):371–380. doi: 10.1093/genetics/91.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON E. J., PECK H. D., Jr COUPLING OF PHOSPHORYLATION AND CARBON DIOXIDE FIXATION IN EXTRACTS OF THIOBACILLUS THIOPARUS. J Bacteriol. 1965 Apr;89:1041–1050. doi: 10.1128/jb.89.4.1041-1050.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. J. Occurrence of the adenosine monophosphate inhibition of carbon dioxide fixation in photosynthetic and chemosynthetic autotrophs. Arch Biochem Biophys. 1966 Apr;114(1):178–183. doi: 10.1016/0003-9861(66)90319-5. [DOI] [PubMed] [Google Scholar]
- Kleckner N., Roth J., Botstein D. Genetic engineering in vivo using translocatable drug-resistance elements. New methods in bacterial genetics. J Mol Biol. 1977 Oct 15;116(1):125–159. doi: 10.1016/0022-2836(77)90123-1. [DOI] [PubMed] [Google Scholar]
- Knauf V. C., Nester E. W. Wide host range cloning vectors: a cosmid clone bank of an Agrobacterium Ti plasmid. Plasmid. 1982 Jul;8(1):45–54. doi: 10.1016/0147-619x(82)90040-3. [DOI] [PubMed] [Google Scholar]
- Krishnapillai V., Royle P., Lehrer J. Insertions of the transposon Tn1 into the Pseudomonas aeruginosa chromosome. Genetics. 1981 Mar-Apr;97(3-4):495–511. doi: 10.1093/genetics/97.3-4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuner J. M., Kaiser D. Introduction of transposon Tn5 into Myxococcus for analysis of developmental and other nonselectable mutants. Proc Natl Acad Sci U S A. 1981 Jan;78(1):425–429. doi: 10.1073/pnas.78.1.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léjohn H. B., Van Caeseele L., Lees H. Catabolite repression in the facultative chemoautotroph Thiobacillus novellus. J Bacteriol. 1967 Nov;94(5):1484–1491. doi: 10.1128/jb.94.5.1484-1491.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin A. Organic nutrition of chemolithotrophic bacteria. Annu Rev Microbiol. 1978;32:433–468. doi: 10.1146/annurev.mi.32.100178.002245. [DOI] [PubMed] [Google Scholar]
- PECK H. D., Jr Symposium on metabolism of inorganic compounds. V. Comparative metabolism of inorganic sulfur compounds in microorganisms. Bacteriol Rev. 1962 Mar;26:67–94. doi: 10.1128/br.26.1.67-94.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinto M., Bender R. A. Use of bacteriophage P1 as a vector for Tn5 insertion mutagenesis. Appl Environ Microbiol. 1984 Feb;47(2):436–438. doi: 10.1128/aem.47.2.436-438.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B. F., Hoare D. S. New facultative Thiobacillus and a reevaluation of the heterotrophic potential of Thiobacillus novellus. J Bacteriol. 1969 Oct;100(1):487–497. doi: 10.1128/jb.100.1.487-497.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson J. A., Hendson M., Magnes R. M. Mutagenesis by insertion of drug resistance transposon Tn7 into a vibrio species. J Bacteriol. 1981 Oct;148(1):374–378. doi: 10.1128/jb.148.1.374-378.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M., Harayama S., Iino T. Tn501 insertion mutagenesis in Pseudomonas aeruginosa PAO. Mol Gen Genet. 1984;196(3):494–500. doi: 10.1007/BF00436198. [DOI] [PubMed] [Google Scholar]
- Weaver K. E., Tabita F. R. Isolation and partial characterization of Rhodopseudomonas sphaeroides mutants defective in the regulation of ribulose bisphosphate carboxylase/oxygenase. J Bacteriol. 1983 Nov;156(2):507–515. doi: 10.1128/jb.156.2.507-515.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Falkow S. Transposon insertion and subsequent donor formation promoted by Tn501 in Bordetella pertussis. J Bacteriol. 1983 Jan;153(1):304–309. doi: 10.1128/jb.153.1.304-309.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood A. P., Kelly D. P., Thurston C. F. Simultaneous operation of three catabolic pathways in the metabolism of glucose by Thiobacillus A2. Arch Microbiol. 1977 Jun 20;113(3):265–274. doi: 10.1007/BF00492034. [DOI] [PubMed] [Google Scholar]


