Abstract
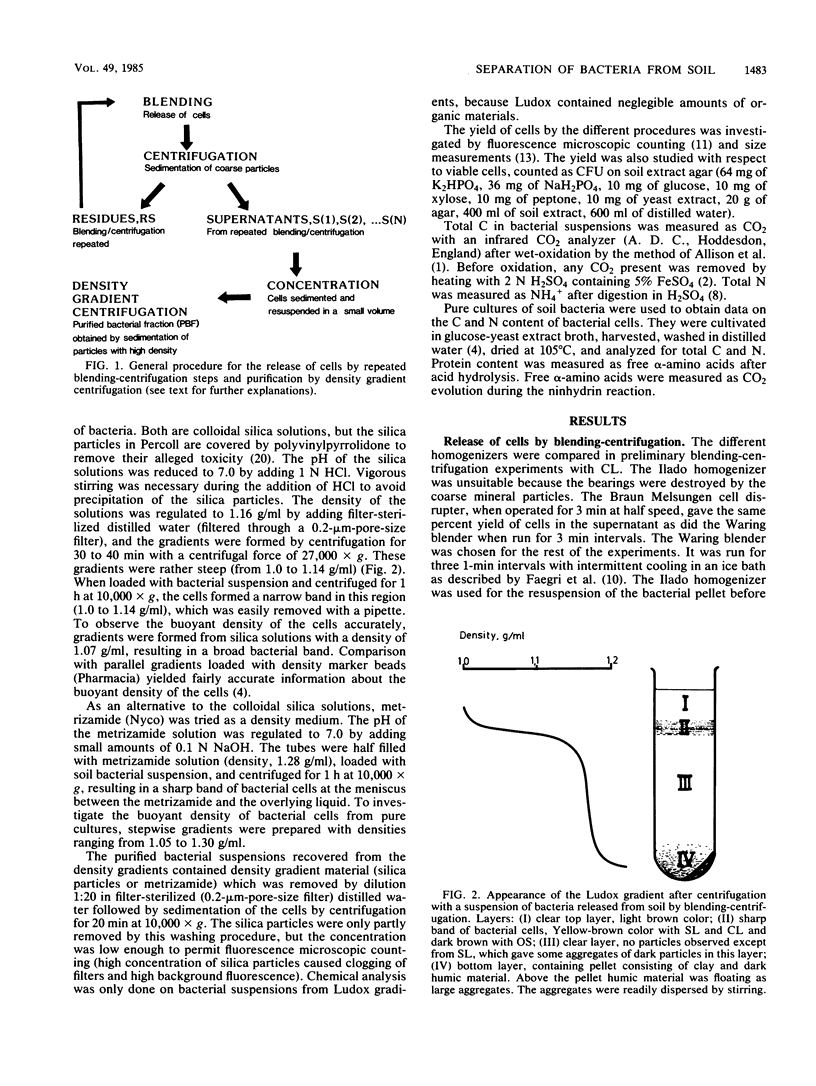
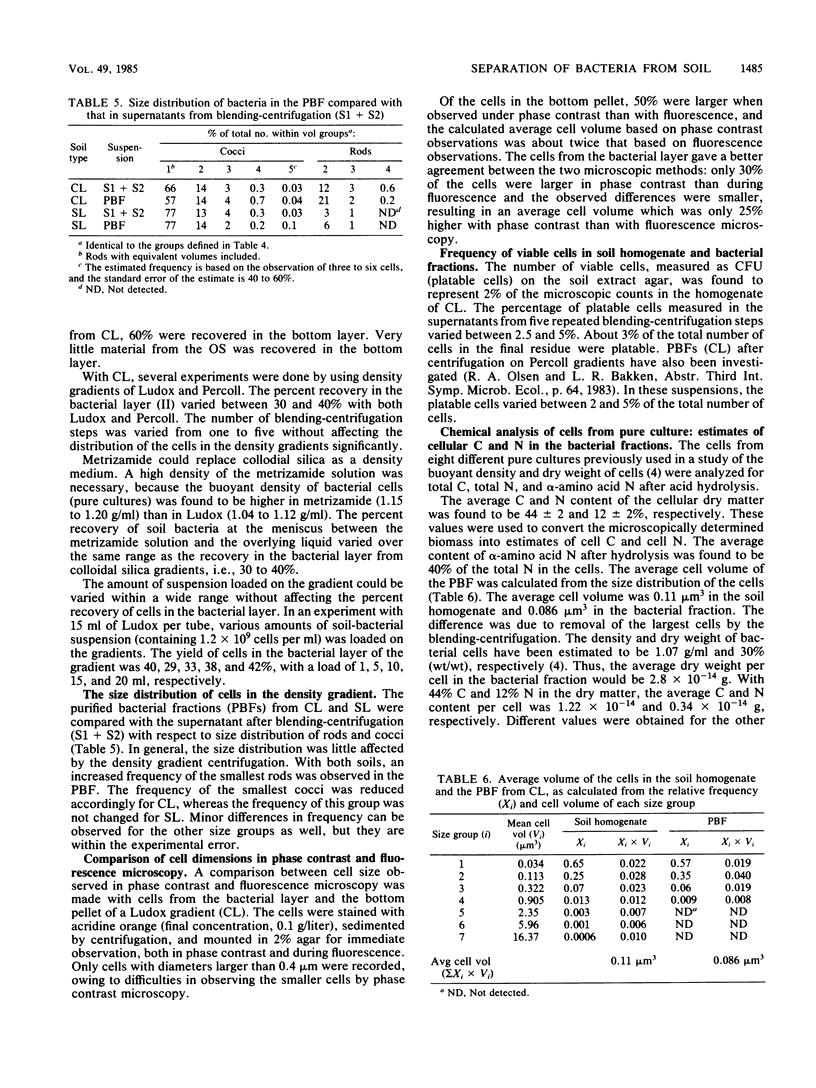
Bacteria were released and separated from soil by a simple blending-centrifugation procedure. The percent yield of bacterial cells (microscopic counts) in the supernatants varied over a wide range depending on the soil type. The superantants contained large amounts of noncellular organic material and clay particles. Further purification of the bacterial cells was obtained by centrifugation in density gradients, whereby the clay particles and part of the organic materials sedimented. A large proportion of the bacteria also sedimented through the density gradient, showing that they had a buoyant density above 1.2 g/ml. Attachment to clay minerals and humic material may account for this apparently high buoyant density. The percent yield of cells was negatively correlated with the clay content of the soils, whereas the purity was positively correlated with it. The cell size distribution and the relative frequency of colony-forming cells were similar in the soil homogenate, the supernatants after blending-centrifugation, and the purified bacterial fraction. In purified bacterial fraction from a clay loam, the microscopically measured biomass could account for 20 to 25% of the total C and 30 to 40% of the total N as cellular C and N. The amount of cellular C and N may be higher, however, owing to an underestimation of the cell diameter during fluorescence. A part of the contamination could be ascribed to extracellular structures as well as partly decayed cells, which were not revealed by fluorescence microscopy.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bae H. C., Cota-Robles E. H., Casida L. E. Microflora of soil as viewed by transmission electron microscopy. Appl Microbiol. 1972 Mar;23(3):637–648. doi: 10.1128/am.23.3.637-648.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakken L. R., Olsen R. A. Buoyant densities and dry-matter contents of microorganisms: conversion of a measured biovolume into biomass. Appl Environ Microbiol. 1983 Apr;45(4):1188–1195. doi: 10.1128/aem.45.4.1188-1195.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill D. L., Rucinsky T. E., Casida L. E., Jr Release of microorganisms from soil with respect to transmission electron microscopy viewing and plate counts. Antonie Van Leeuwenhoek. 1977;43(1):73–87. doi: 10.1007/BF02316212. [DOI] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Verma S. B. Zur Problem des quantitativen Nachweises der Mikroflora des Bodens mit der Methode Koch. I. Einfluss der verschiedenen Dispergierungsmittel und Verdünnungsmedien auf Bodendispersion und Gesamtkeizahl. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg. 1968 Jul;122(4):357–385. [PubMed] [Google Scholar]
- Wolff D. A. The separation of cells and subcellular particles by colloidal silica density gradient centrifugation. Methods Cell Biol. 1975;10:85–104. doi: 10.1016/s0091-679x(08)60731-1. [DOI] [PubMed] [Google Scholar]
- Wollum A. G., Miller R. H. Density Centrifugation Method for Recovering Rhizobium spp. from Soil for Fluorescent-Antibody Studies. Appl Environ Microbiol. 1980 Feb;39(2):466–469. doi: 10.1128/aem.39.2.466-469.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


