Abstract
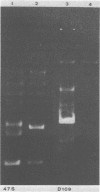
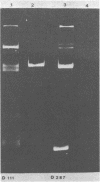
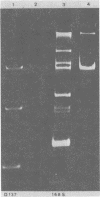
Sixteen strains of Lactobacillus reuteri and 20 strains of Lactobacillus acidophilus were tested for resistance to 22 antibiotics by using commercially available sensitivity disks. Evidence suggesting linkage of these resistances to plasmids was obtained by "curing" experiments with acridine dyes and high growth temperatures. Examination of plasmid patterns of agarose gel electrophoresis provided further evidence of loss in plasmid DNA under curing conditions in some of the strains examined.
Full text
PDF
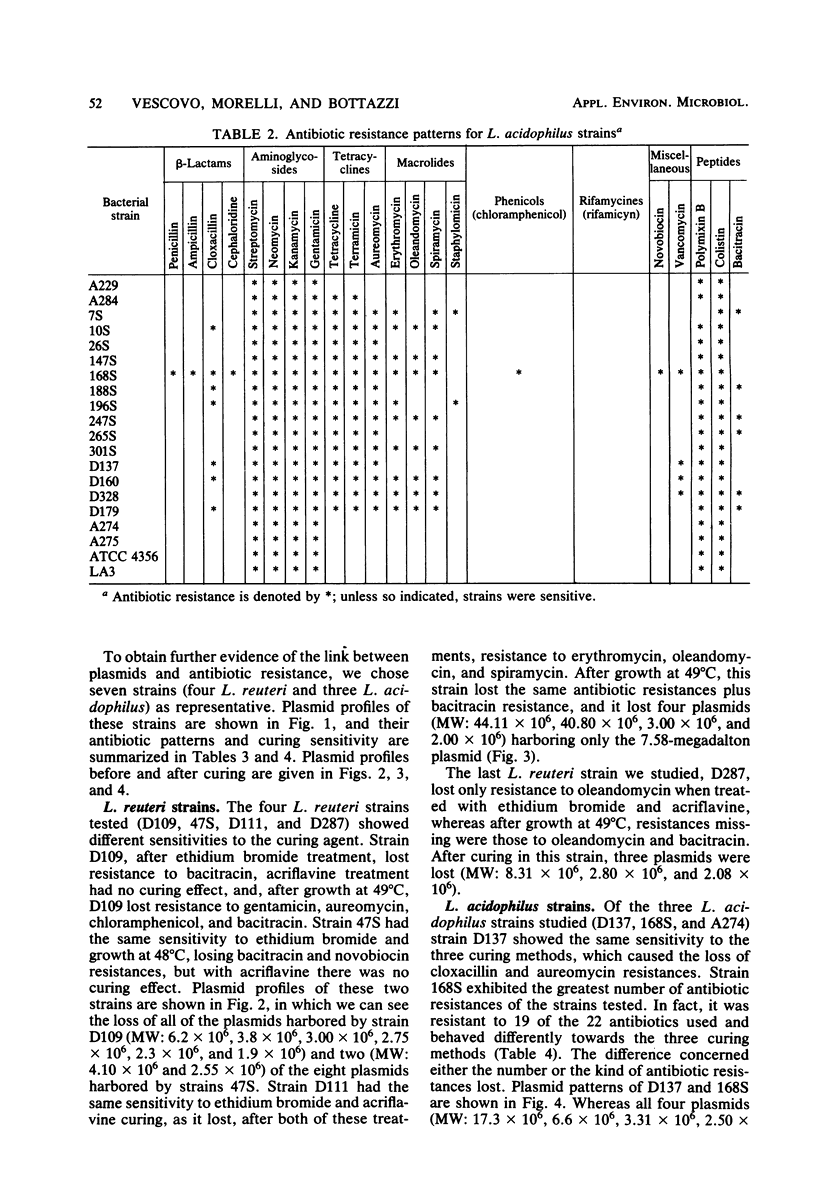
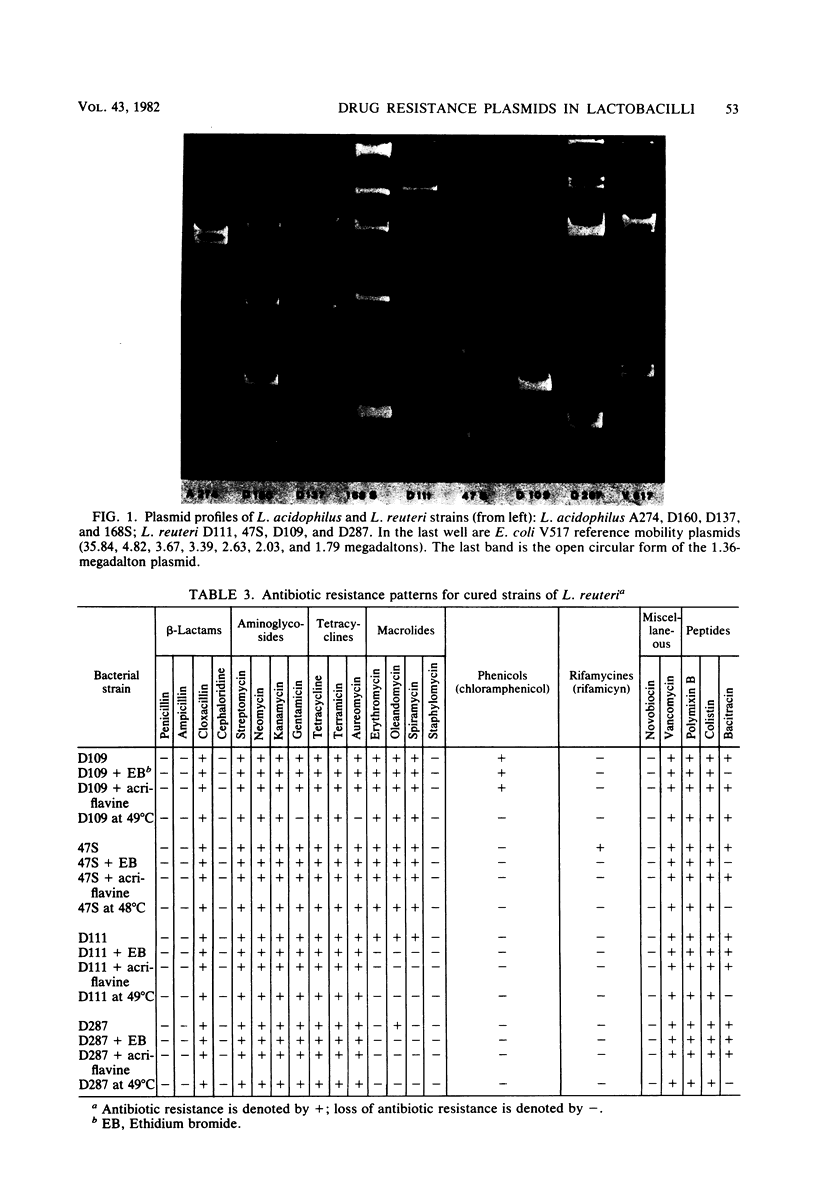
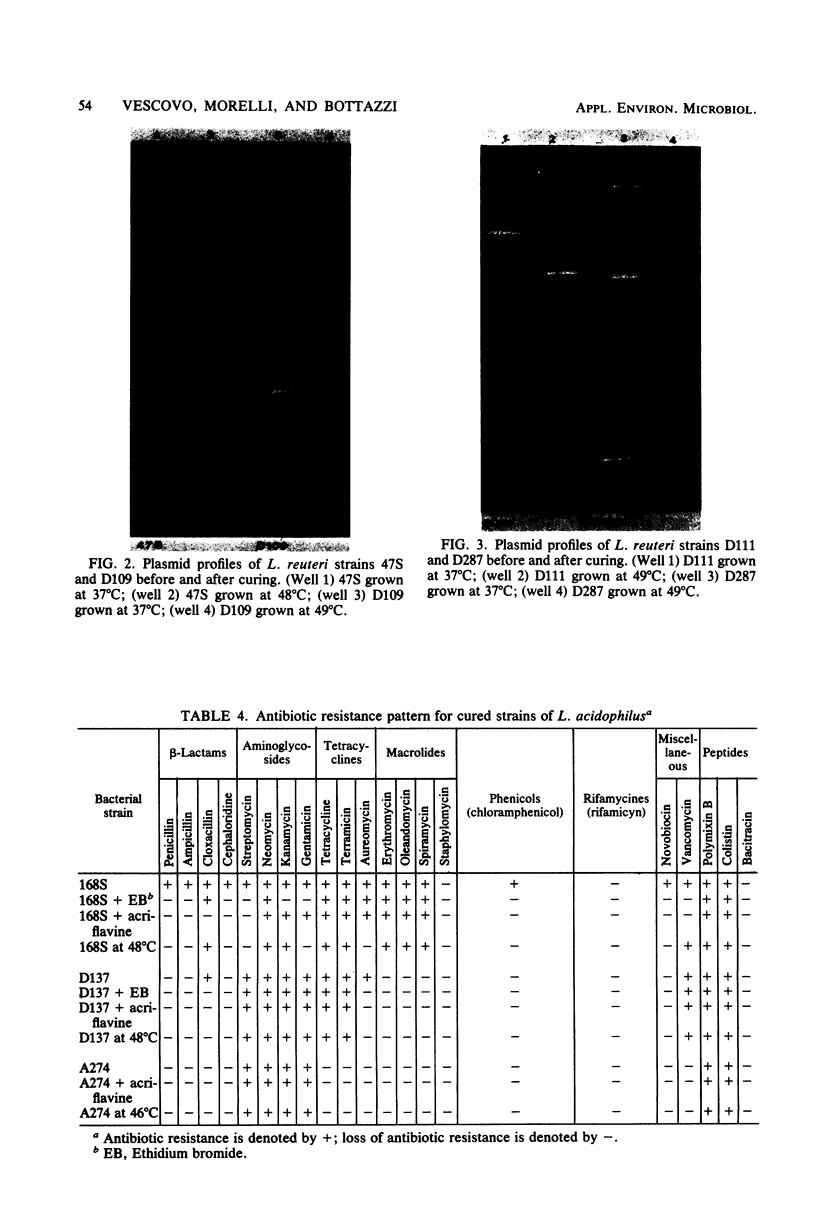


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouanchaud D. H., Scavizzi M. R., Chabbert Y. A. Elimination by ethidium bromide of antibiotic resistance in enterobacteria and staphylococci. J Gen Microbiol. 1968 Dec;54(3):417–425. doi: 10.1099/00221287-54-3-417. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Clowes R. C. Molecular structure of bacterial plasmids. Bacteriol Rev. 1972 Sep;36(3):361–405. doi: 10.1128/br.36.3.361-405.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier T. C., Nester E. W. Isolation of covalently closed circular DNA of high molecular weight from bacteria. Anal Biochem. 1976 Dec;76(2):431–441. doi: 10.1016/0003-2697(76)90338-9. [DOI] [PubMed] [Google Scholar]
- Klaenhammer T. R., Sutherland S. M. Detection of plasmid deoxyribonucleic acid in an isolate of Lactobacillus acidophilus. Appl Environ Microbiol. 1980 Mar;39(3):671–674. doi: 10.1128/aem.39.3.671-674.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAY J. W., HOUGHTON R. H., PERRET C. J. THE EFFECT OF GROWTH AT ELEVATED TEMPERATURES ON SOME HERITABLE PROPERTIES OF STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1964 Nov;37:157–169. doi: 10.1099/00221287-37-2-157. [DOI] [PubMed] [Google Scholar]
- Macrina F. L., Kopecko D. J., Jones K. R., Ayers D. J., McCowen S. M. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978 Jun;1(3):417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A., Zottola E. A. Loss of lactose metabolism in lactic streptococci. Appl Microbiol. 1972 Jun;23(6):1090–1096. doi: 10.1128/am.23.6.1090-1096.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi S. Drug resistance plasmids. Mol Cell Biochem. 1979 Aug 15;26(3):135–181. doi: 10.1007/BF00423044. [DOI] [PubMed] [Google Scholar]
- Mitsuhashi S. Epidemiological and genetical study of drug resistance in Staphylococcus aureus. Jpn J Microbiol. 1967 Mar;11(1):49–68. doi: 10.1111/j.1348-0421.1967.tb00320.x. [DOI] [PubMed] [Google Scholar]
- Novick R. P. Penicillinase plasmids of Staphylococcus aureus. Fed Proc. 1967 Jan-Feb;26(1):29–38. [PubMed] [Google Scholar]
- Rozek C. E., Timberlake W. E. Restriction endonuclease mapping by crossed contact hybridization: the ribosomal RNA genes of Achlya ambisexualis. Nucleic Acids Res. 1979 Nov 24;7(6):1567–1578. doi: 10.1093/nar/7.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarra P. G., Magri M., Bottazzi V., Dellaglio F. Genetic heterogeneity among Lactobacillus acidophilus strains. Antonie Van Leeuwenhoek. 1980;46(2):169–176. doi: 10.1007/BF00444072. [DOI] [PubMed] [Google Scholar]
- Scioli C., Esposito S., Anzilotti G., Pavone A., Pennucci C. Antibiotico-resistenza e trasferimenti nelle enterobacteriaceae di provenienza avicola. Boll Ist Sieroter Milan. 1980 Mar 31;59(1):4–11. [PubMed] [Google Scholar]
- Sozzi T., Smiley M. B. Antibiotic Resistances of Yogurt Starter Cultures Streptococcus thermophilus and Lactobacillus bulgaricus. Appl Environ Microbiol. 1980 Nov;40(5):862–865. doi: 10.1128/aem.40.5.862-865.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terawaki Y., Takayasu H., Akiba T. Thermosensitive replication of a kanamycin resistance factor. J Bacteriol. 1967 Sep;94(3):687–690. doi: 10.1128/jb.94.3.687-690.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T., FUKASAWA T. Episome-mediated transfer of drug resistance in Enterobacteriaceae. II. Elimination of resistance factors with acridine dyes. J Bacteriol. 1961 May;81:679–683. doi: 10.1128/jb.81.5.679-683.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T. Evolutionary relationships of R factors with other episomes and plasmids. Fed Proc. 1967 Jan-Feb;26(1):23–28. [PubMed] [Google Scholar]