Abstract
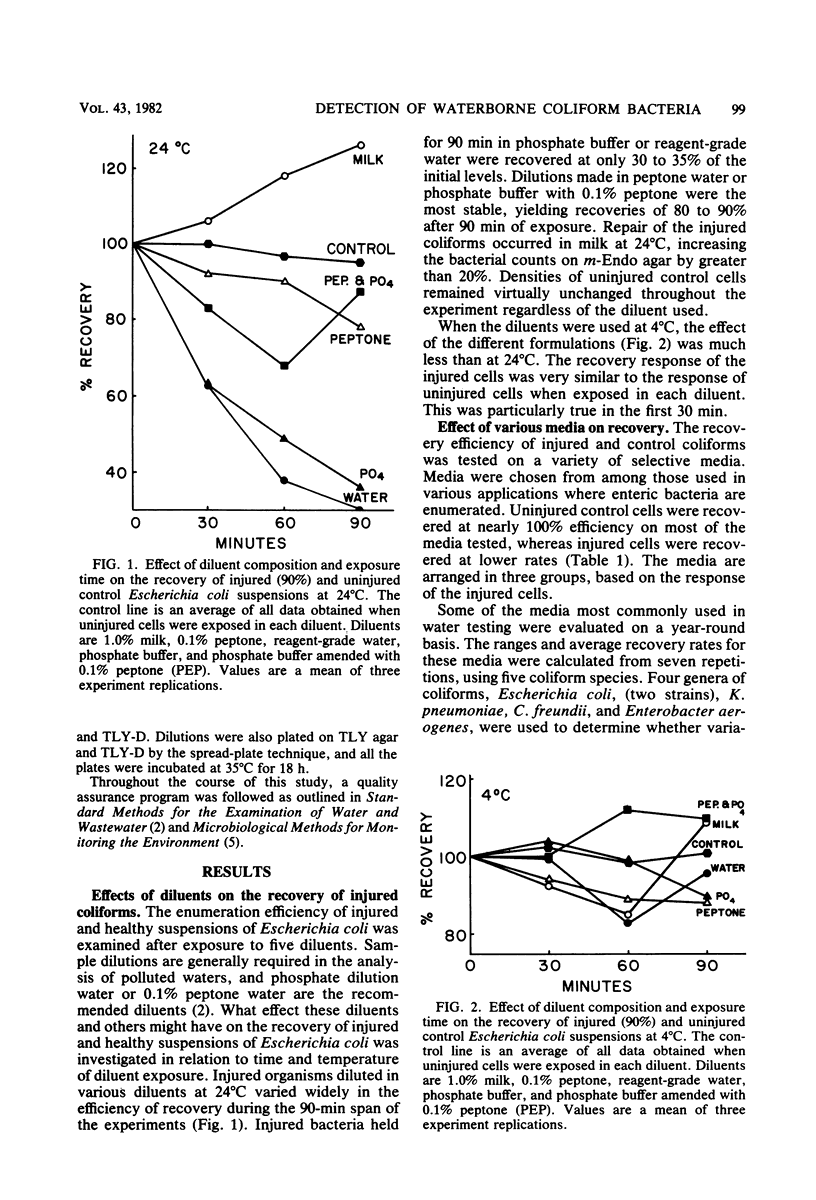
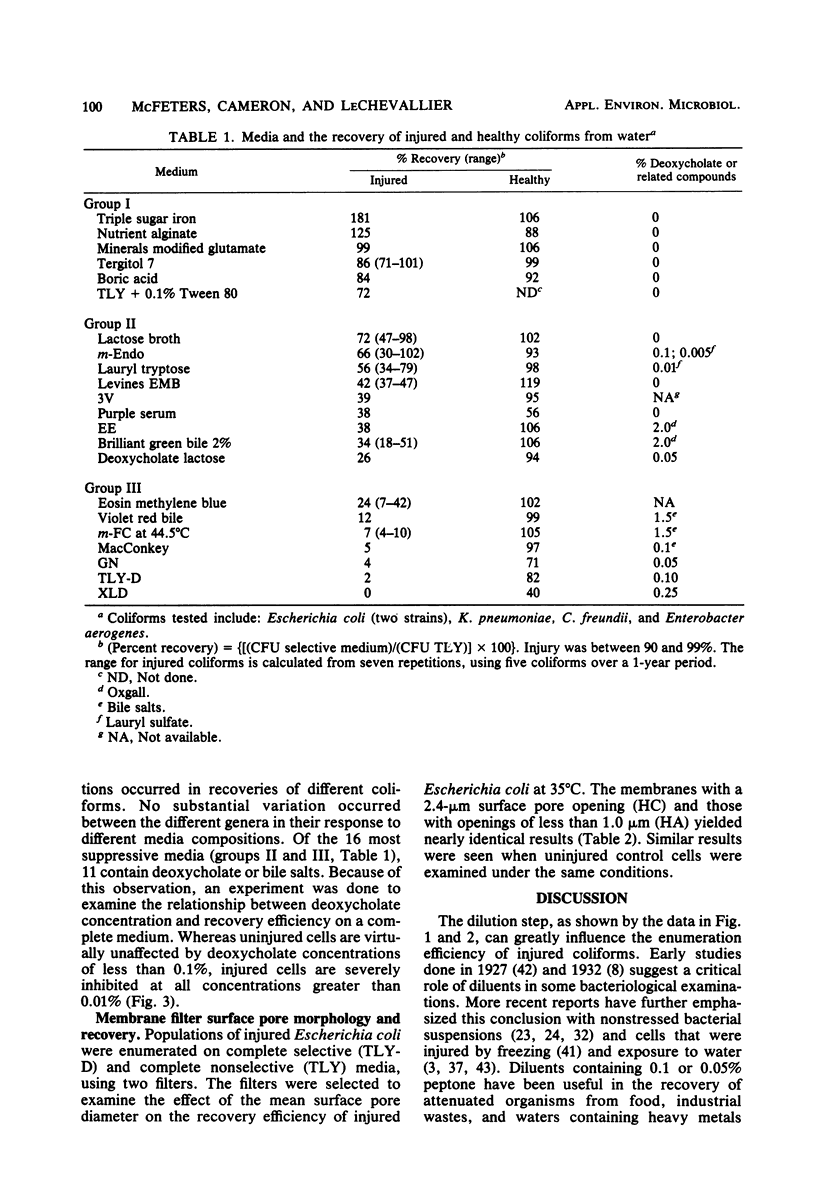
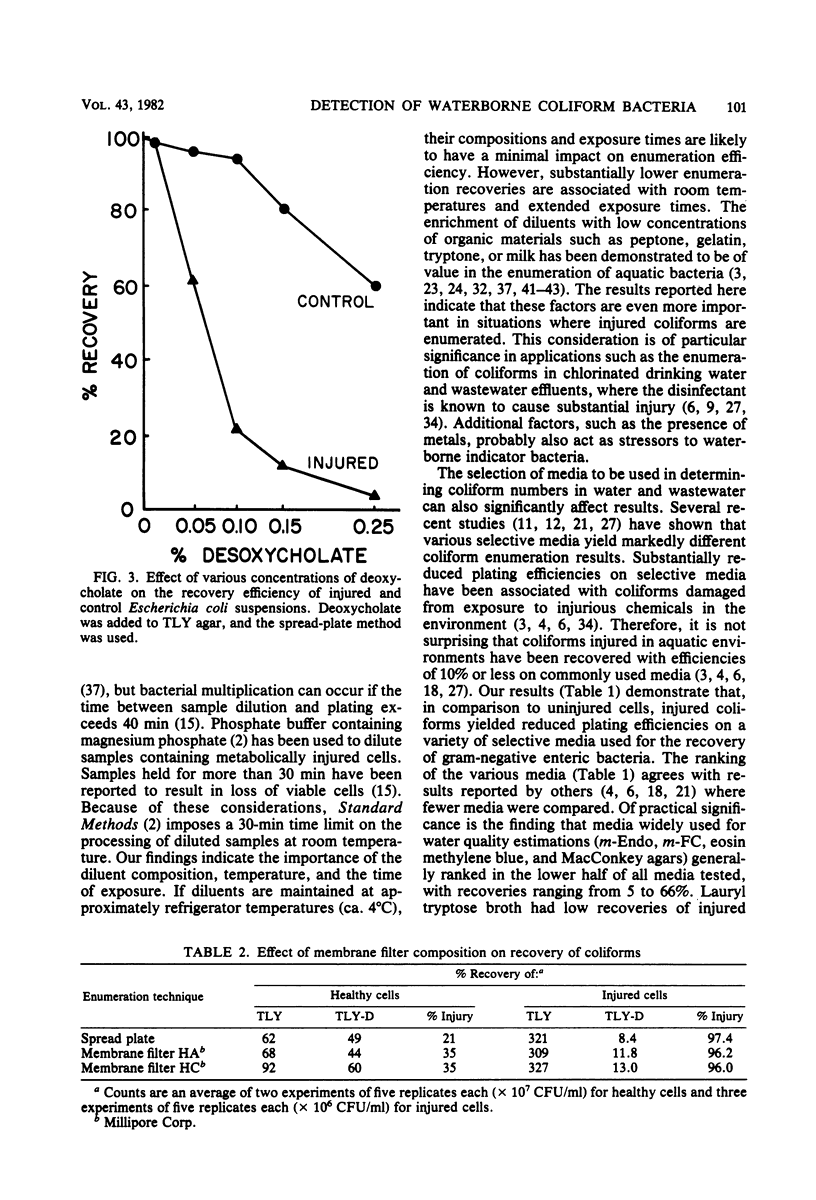
Pure cultures of Escherichia coli, Klebsiella pneumoniae, Enterobacter aerogenes, and Citrobacter freundii were injured ( greater than 90%) in water from a dead-end section of the Bozeman, Montana, distribution system. The effects of the following laboratory variables on the enumeration efficiency of injured and undamaged control cells were examined: (i) diluent composition, temperature, and time of exposure; (ii) media, using various formulations employed in enumerating gram-negative bacteria; and (iii) surface pore morphology of membrane filters. The addition of peptone or milk solids to diluents and low temperature (4 degrees C) maximized the recovery of injured cells, but had little effect on undamaged cells. Control cells were recovered with high efficiencies on most media tested, but recoveries of injured cells ranged from 0 to near 100%. Most of the media commonly used in water analysis recovered less than 30% of injured cells. This was explained in part by the sensitivity of injured bacteria to deoxycholate concentrations greater than 0.01%, whereas control cells were unaffected by 0.1%. Membrane filter surface pore morphology (at 35 degrees C) had a negligible effect on total coliform recoveries. Recommendations are made regarding procedures to improve the recovery of injured coliforms by routine laboratory practices.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN L. A., PASLEY S. M., PIERCE M. S. F. Conditions affecting the growth of Bacterium coli on bile salts media; enumeration of this organism in polluted waters. J Gen Microbiol. 1952 Nov;7(3-4):257–267. doi: 10.1099/00221287-7-3-4-257. [DOI] [PubMed] [Google Scholar]
- Bissonnette G. K., Jezeski J. J., McFeters G. A., Stuart D. G. Evaluation of recovery methods to detect coliforms in water. Appl Environ Microbiol. 1977 Mar;33(3):590–595. doi: 10.1128/aem.33.3.590-595.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonnette G. K., Jezeski J. J., McFeters G. A., Stuart D. G. Influence of environmental stress on enumeration of indicator bacteria from natural waters. Appl Microbiol. 1975 Feb;29(2):186–194. doi: 10.1128/am.29.2.186-194.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braswell J. R., Hoadley A. W. Recovery of Escherichia coli from chlorinated secondary sewage. Appl Microbiol. 1974 Aug;28(2):328–329. doi: 10.1128/am.28.2.328-329.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield C. T. The Selection of a Dilution Water for Bacteriological Examinations. J Bacteriol. 1932 May;23(5):355–368. doi: 10.1128/jb.23.5.355-368.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camper A. K., McFeters G. A. Chlorine injury and the enumeration of waterborne coliform bacteria. Appl Environ Microbiol. 1979 Mar;37(3):633–641. doi: 10.1128/aem.37.3.633-641.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutkaand B. J., Tobin S. E. Study on the efficiency of four procedures for enumerating coliforms in water. Can J Microbiol. 1976 May;22(5):630–635. doi: 10.1139/m76-093. [DOI] [PubMed] [Google Scholar]
- Evans T. M., Seidler R. J., LeChevallier M. W. Impact of verification media and resuscitation on accuracy of the membrane filter total coliform enumeration technique. Appl Environ Microbiol. 1981 May;41(5):1144–1151. doi: 10.1128/aem.41.5.1144-1151.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabow W. O., du Preez M. Comparison of m-Endo LES, MacConkey, and Teepol media for membrane filtration counting of total coliform bacteria in water. Appl Environ Microbiol. 1979 Sep;38(3):351–358. doi: 10.1128/aem.38.3.351-358.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B. L., Clausen E. M., Litsky W. Two-temperature membrane filter method for enumerating fecal coliform bacteria from chlorinated effluents. Appl Environ Microbiol. 1977 Jun;33(6):1259–1264. doi: 10.1128/aem.33.6.1259-1264.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoadley A. W., Cheng C. M. The recovery of indicator bacteria on selective media. J Appl Bacteriol. 1974 Mar;37(1):45–57. doi: 10.1111/j.1365-2672.1974.tb00413.x. [DOI] [PubMed] [Google Scholar]
- Lin S. D. Evaluation of millipore HA and HC membrane filters for the enumeration of indicator bacteria. Appl Environ Microbiol. 1976 Aug;32(2):300–302. doi: 10.1128/aem.32.2.300-302.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. D. Membrane filter method for recovery of fecal coliforms in chlorinated sewage effluents. Appl Environ Microbiol. 1976 Oct;32(4):547–552. doi: 10.1128/aem.32.4.547-552.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFeters G. A., Stuart D. G. Survival of coliform bacteria in natural waters: field and laboratory studies with membrane-filter chambers. Appl Microbiol. 1972 Nov;24(5):805–811. doi: 10.1128/am.24.5.805-811.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presswood W. G., Brown L. R. Comparison of Gelman and Millipore membrane filters for enumerating fecal coliform bacteria. Appl Microbiol. 1973 Sep;26(3):332–336. doi: 10.1128/am.26.3.332-336.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray B., Speck M. L. Discrepancies in the enumeration of Escherichia coli. Appl Microbiol. 1973 Apr;25(4):494–498. doi: 10.1128/am.25.4.494-498.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose R. E., Geldreich E. E., Litsky W. Improved membrane filter method for fecal coliform analysis. Appl Microbiol. 1975 Apr;29(4):532–536. doi: 10.1128/am.29.4.532-536.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIPE E. L., Jr, CAMERON G. M. A comparison of the membrane filter with the most probable number method for coliform determinations from several waters. Appl Microbiol. 1954 Mar;2(2):85–88. doi: 10.1128/am.2.2.85-88.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRAKA R. P., STOKES J. L. Rapid destruction of bacteria in commonly used diluents and its elimination. Appl Microbiol. 1957 Jan;5(1):21–25. doi: 10.1128/am.5.1.21-25.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheusner D. L., Busta F. F., Speck M. L. Inhibition of injured Escherichia coli by several selective agents. Appl Microbiol. 1971 Jan;21(1):46–49. doi: 10.1128/am.21.1.46-49.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek K. J., Suslavich R. V., Sohn B. I., Dawson F. W. Optimum membrane structures for growth of coliform and fecal coliform organisms. Appl Microbiol. 1975 Oct;30(4):685–691. doi: 10.1128/am.30.4.685-691.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart D. G., McFeters G. A., Schillinger J. E. Membrane filter technique for the quantification of stressed fecal coliforms in the aquatic environment. Appl Environ Microbiol. 1977 Jul;34(1):42–46. doi: 10.1128/aem.34.1.42-46.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin R. S., Dutka B. J. Comparison of the surface structure, metal binding, and fecal coliform recoveries of nine membrane filters. Appl Environ Microbiol. 1977 Jul;34(1):69–79. doi: 10.1128/aem.34.1.69-79.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin R. S., Lomax P., Kushner D. J. Comparison of nine brands of membrane filter and the most-probable-number methods for total coliform enumeration in sewage-contaminated drinking water. Appl Environ Microbiol. 1980 Aug;40(2):186–191. doi: 10.1128/aem.40.2.186-191.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler W. A., Hartsell S. E. Diluent composition and the recovery of Escherichia coli. Appl Microbiol. 1969 Nov;18(5):956–957. doi: 10.1128/am.18.5.956-957.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow C. E., Brooke O. R. THE VIABILITY OF VARIOUS SPECIES OF BACTERIA IN AQUEOUS SUSPENSIONS. J Bacteriol. 1927 Apr;13(4):235–243. doi: 10.1128/jb.13.4.235-243.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S. Y., Klein D. A. Starvation effects on Escherichia coli and aquatic bacterial responses to nutrient addition and secondary warming stresses. Appl Environ Microbiol. 1976 Feb;31(2):216–220. doi: 10.1128/aem.31.2.216-220.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaske S. K., Dockins W. S., McFeters G. A. Cell envelope damage in Escherichia coli caused by short-term stress in water. Appl Environ Microbiol. 1980 Aug;40(2):386–390. doi: 10.1128/aem.40.2.386-390.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


