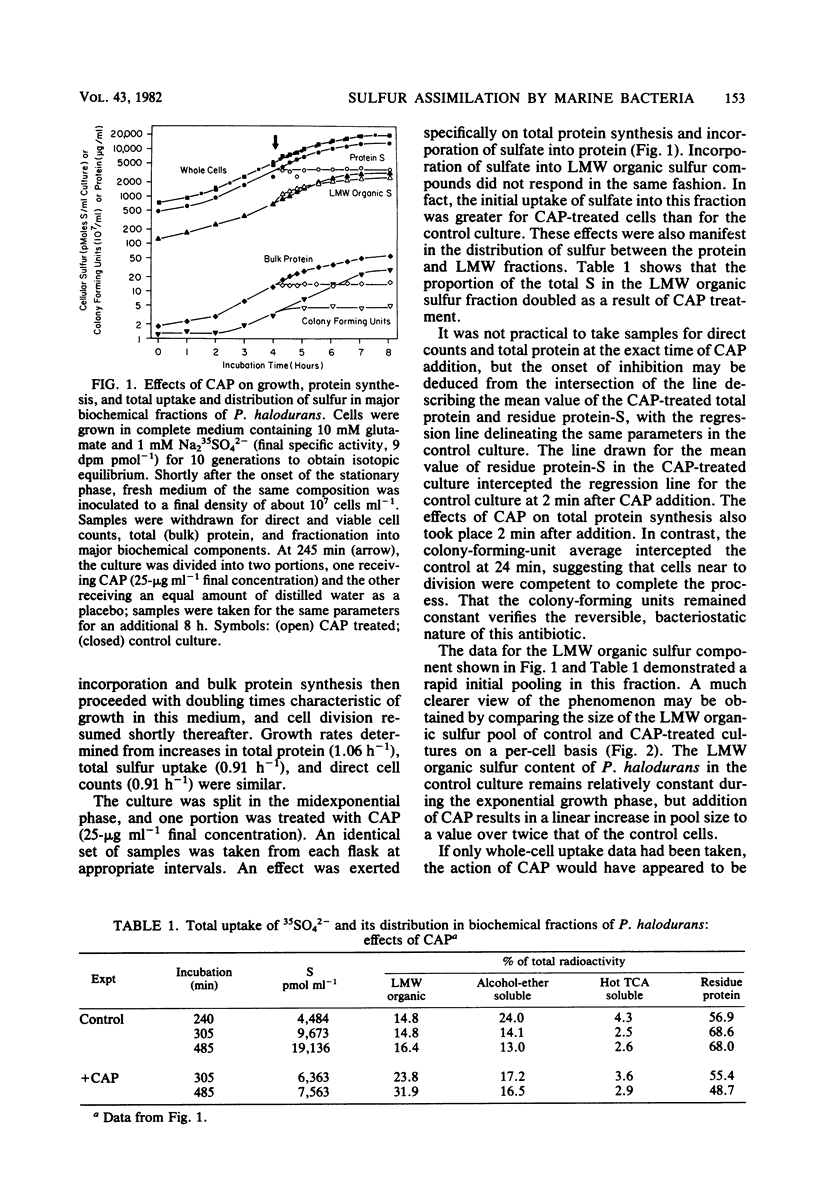
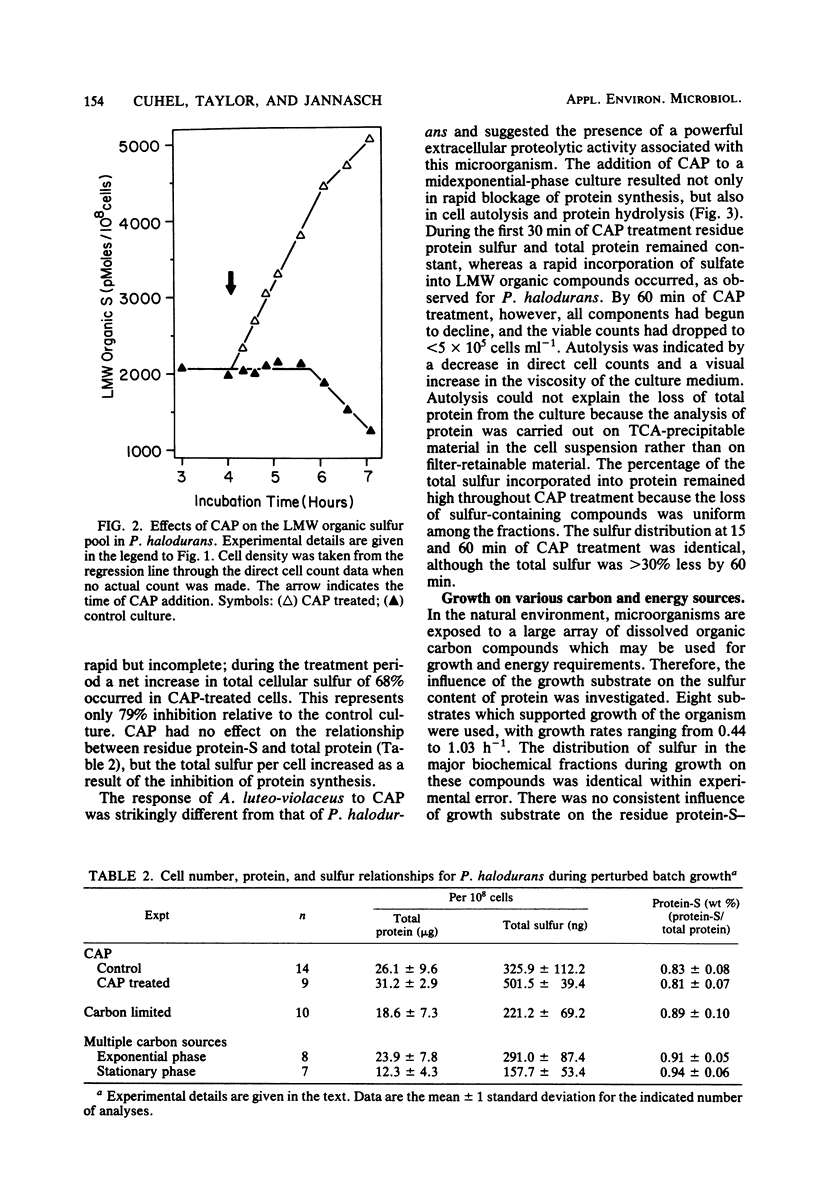
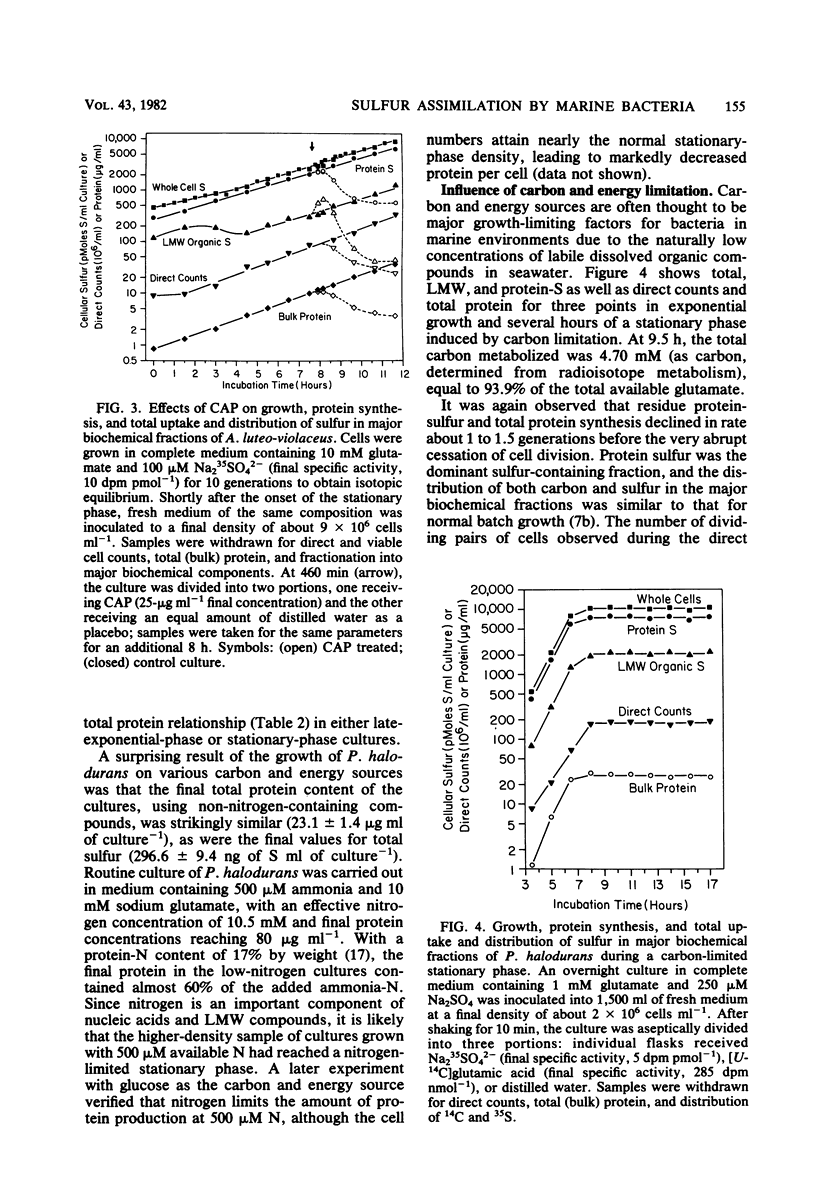
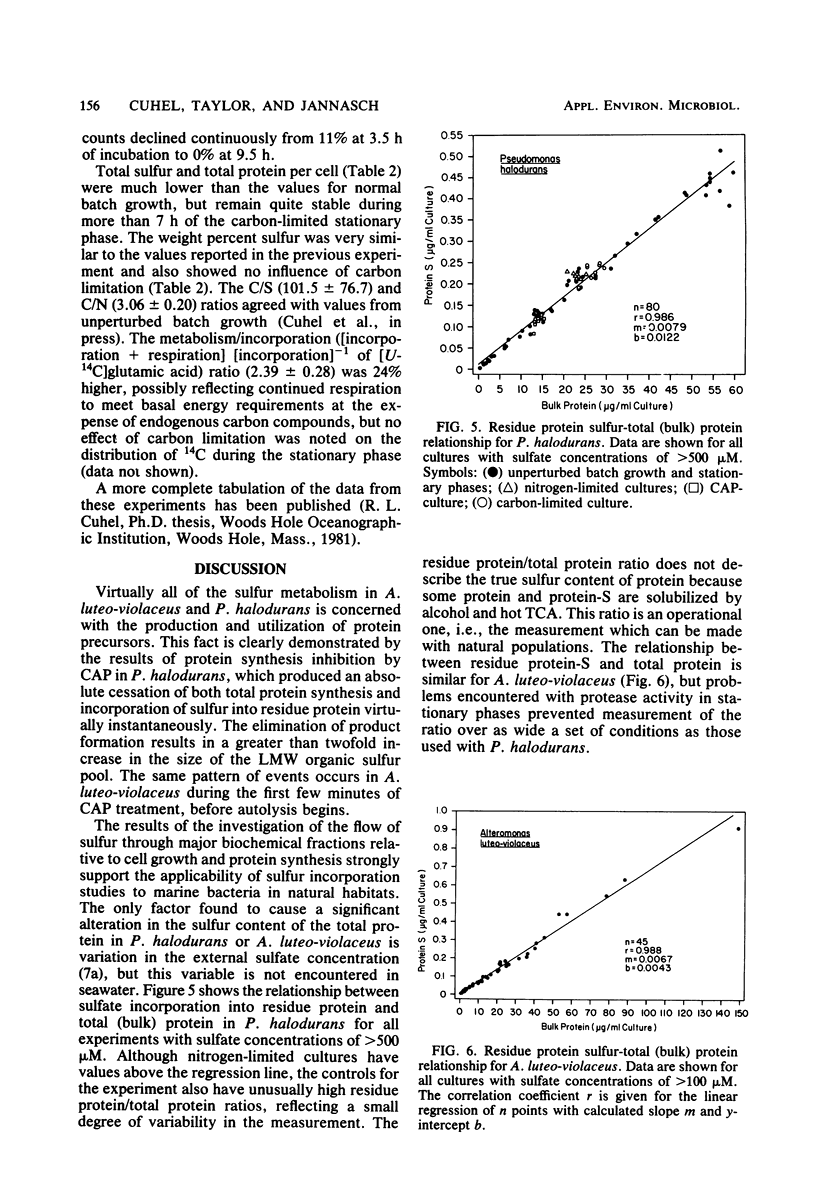
Abstract
The antibiotic protein synthesis inhibitor chloramphenicol specifically blocked the incorporation of [35S]sulfate into the residue protein of two marine bacteria, Pseudomonas halodurans and Alteromonas luteo-violaceus. Simultaneous inhibition of total protein synthesis occurred, but incorporation of 35S into low-molecular-weight organic compounds continued. A. luteo-violaceus rapidly autolyzed, with similar reduction in cell counts, total culture protein and cellular sulfur, whereas P. halodurans remained viable. Treatment with chloramphenicol, growth during nitrogen and carbon limitation, and the carbon and energy sources used for growth did not alter the sulfur content of P. halodurans protein. The mean value (1.09%, by weight), representing a wide variety of environmentally relevant growth conditions, was in agreement with model protein composition. The variability of cellular composition of P. halodurans and A. luteo-violaceus is discussed with respect to the measurement of bacterial growth in natural environments. Total carbon and nitrogen per cell varied greatly (coefficient of variation, ca. 100%) depending on growth conditions. Variation in total sulfur and protein per cell was much less (coefficient of variation, <50%), but the least variation was found for sulfate incorporation into residue protein (coefficient of variation, ca. 15%). Thus, sulfate incorporation into residue protein can be used as an accurate measurement of de novo protein synthesis in these bacteria.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anfinsen C. B. Principles that govern the folding of protein chains. Science. 1973 Jul 20;181(4096):223–230. doi: 10.1126/science.181.4096.223. [DOI] [PubMed] [Google Scholar]
- Antoine A. D., Tepper B. S. Environmental control of glycogen and lipid content of Mycobacterium phlei. J Gen Microbiol. 1969 Feb;55(2):217–226. doi: 10.1099/00221287-55-2-217. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Campbell P. G., Baker J. H. Estimation of bacterial production in fresh waters by the simultaneous measurement of [35S]sulphate and d-[3H]glucose uptake in the dark. Can J Microbiol. 1978 Aug;24(8):939–946. doi: 10.1139/m78-156. [DOI] [PubMed] [Google Scholar]
- Carbon J. A., Hung L., Jones D. S. A reversible oxidative in activation of specific transfer RNA species. Proc Natl Acad Sci U S A. 1965 May;53(5):979–986. doi: 10.1073/pnas.53.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuhel R. L., Taylor C. D., Jannasch H. W. Assimilatory sulfur metabolism in marine microorganisms: characteristics and regulation of sulfate transport in Pseudomonas halodurans and Alteromonas luteo-violaceus. J Bacteriol. 1981 Aug;147(2):340–349. doi: 10.1128/jb.147.2.340-349.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuhel R. L., Taylor C. D., Jannasch H. W. Assimilatory sulfur metabolism in marine microorganisms: considerations for the application of sulfate incorporation into protein as a measurement of natural population protein synthesis. Appl Environ Microbiol. 1982 Jan;43(1):160–168. doi: 10.1128/aem.43.1.160-168.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datko A. H., Mudd S. H., Giovanelli J., Macnicol P. K. Sulfur-containing Compounds in Lemna perpusilla 6746 Grown at a Range of Sulfate Concentrations. Plant Physiol. 1978 Oct;62(4):629–635. doi: 10.1104/pp.62.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes E. A., Senior P. J. The role and regulation of energy reserve polymers in micro-organisms. Adv Microb Physiol. 1973;10:135–266. doi: 10.1016/s0065-2911(08)60088-0. [DOI] [PubMed] [Google Scholar]
- Gauthier M. J. Morphological, physiological, and biochemical characteristics of some violet-pigmented bacteria isolated from seawater. Can J Microbiol. 1976 Feb;22(2):138–149. doi: 10.1139/m76-019. [DOI] [PubMed] [Google Scholar]
- Jeanjean R., Hourmant A., Ducet G. Effet des inhibiteurs de groupes SH sur le transport du phosphate chez Chlorella pyrenoidosa. Biochimie. 1975;57(3):383–390. doi: 10.1016/s0300-9084(75)80315-4. [DOI] [PubMed] [Google Scholar]
- Jordan M. J. On counseling minority students in a university center. J Am Coll Health Assoc. 1974 Dec;23(2):146–150. [PubMed] [Google Scholar]
- Jukes T. H., Holmquist R., Moise H. Amino acid composition of proteins: Selection against the genetic code. Science. 1975 Jul 4;189(4196):50–51. doi: 10.1126/science.237322. [DOI] [PubMed] [Google Scholar]
- Kaback H. R., Barnes E. M., Jr Mechanisms of active transport in isolated membrane vesicles. II. The mechanism of energy coupling between D-lactic dehydrogenase and beta-galactoside transport in membrane preparations from Escherichia coli. J Biol Chem. 1971 Sep 10;246(17):5523–5531. [PubMed] [Google Scholar]
- Lehmann M., Wöber G. Accumulation, mobilization and turn-over of glycogen in the blue-green bacterium Anacystis nidulans. Arch Microbiol. 1976 Dec 1;111(1-2):93–97. doi: 10.1007/BF00446554. [DOI] [PubMed] [Google Scholar]
- Lipsett M. N. The behavior of 4-thiouridine in the E. coli s-RNA molecule. Biochem Biophys Res Commun. 1965 Jul 12;20(2):224–229. doi: 10.1016/0006-291x(65)90350-5. [DOI] [PubMed] [Google Scholar]
- Monheimer R. H. Sulfate uptake as a measure of planktonic microbial production in freshwater ecosystems. Can J Microbiol. 1974 Jun;20(6):825–831. doi: 10.1139/m74-127. [DOI] [PubMed] [Google Scholar]
- Nelson S. O., Glover G. I., Magill C. W. The essentiality of sulfhydryl groups to transport in Neurospora crassa. Arch Biochem Biophys. 1975 Jun;168(2):483–489. doi: 10.1016/0003-9861(75)90278-7. [DOI] [PubMed] [Google Scholar]
- Slepecky R. A., Law J. H. SYNTHESIS AND DEGRADATION OF POLY-beta-HYDROXYBUTYRIC ACID IN CONNECTION WITH SPORULATION OF BACILLUS MEGATERIUM. J Bacteriol. 1961 Jul;82(1):37–42. doi: 10.1128/jb.82.1.37-42.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]


