Abstract
The aquatic filamentous cyanobacteria Anabaena oscillarioides and Trichodesmium sp. reveal specific cellular regions of tetrazolium salt reduction. The effects of localized reduction of five tetrazolium salts on N2 fixation (acetylene reduction), 14CO2 fixation, and 3H2 utilization were examined. During short-term (within 30 min) exposures in A. oscillarioides, salt reduction in heterocysts occurred simultaneously with inhibition of acetylene reduction. Conversely, when salts failed to either penetrate or be reduced in heterocysts, no inhibition of acetylene reduction occurred. When salts were rapidly reduced in vegetative cells, 14CO2 fixation and 3H2 utilization rates decreased, whereas salts exclusively reduced in heterocysts were not linked to blockage of these processes. In the nonheterocystous genus Trichodesmium, the deposition of reduced 2,3,5-triphenyl-2-tetrazolium chloride (TTC) in the internal cores of trichomes occurs simultaneously with a lowering of acetylene reduction rates. Since TTC deposition in heterocysts of A. oscillarioides occurs contemporaneously with inhibition of acetylene reduction, we conclude that the cellular reduction of this salt is of use in locating potential N2-fixing sites in cyanobacteria. The possible applications and problems associated with interpreting localized reduction of tetrazolium salts in cyanobacteria are presented.
Full text
PDF




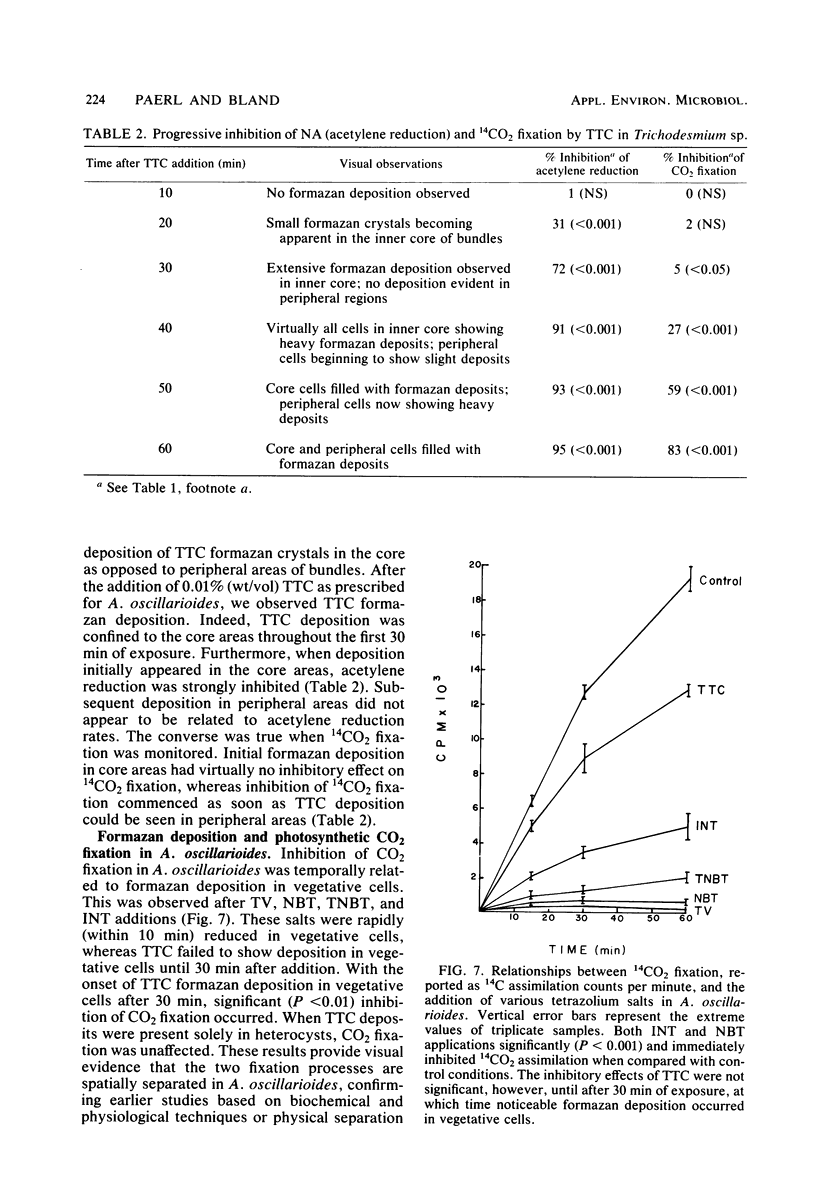
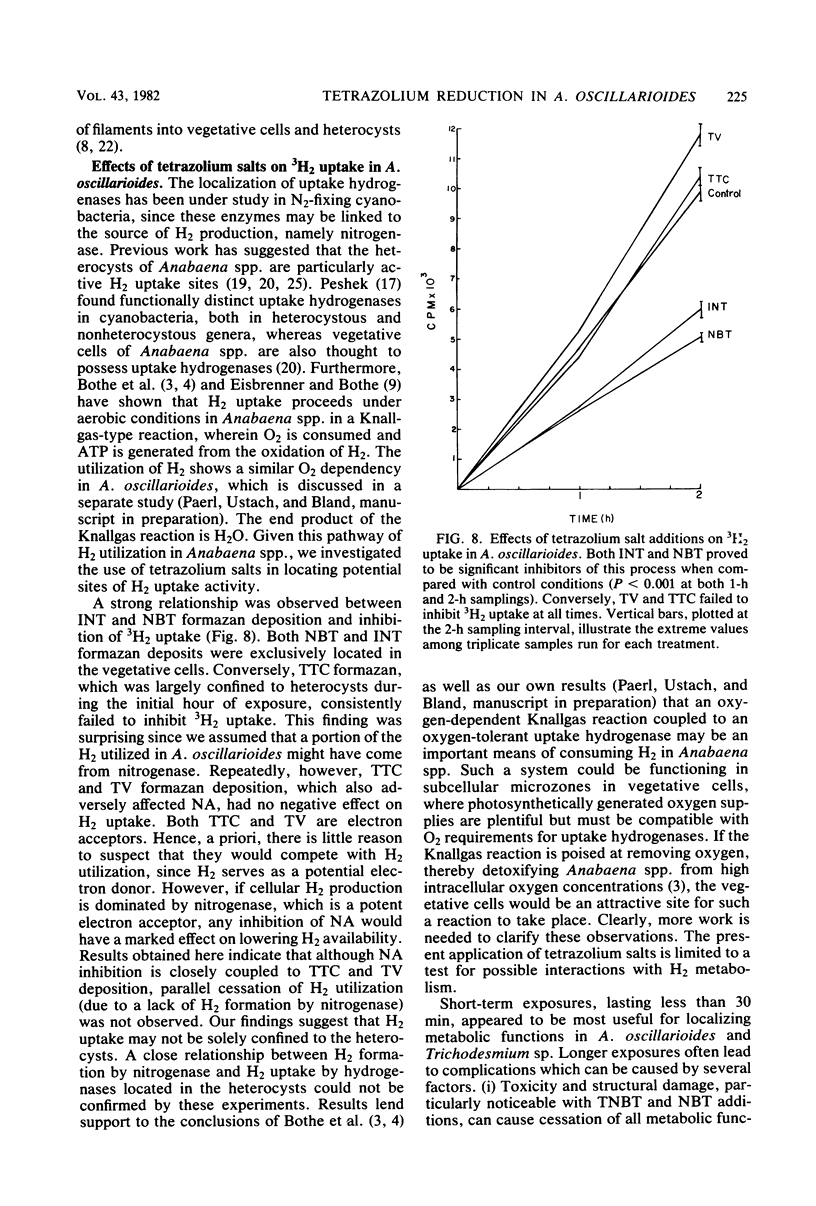

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bisalputra T., Brown D. L., Weier T. E. Possible respiratory sites in a blue-green alga Nostoc sphaericum as demonstrated by potassium tellurite and tetranitro-blue tetrazolium reduction. J Ultrastruct Res. 1969 Apr;27(2):182–197. [PubMed] [Google Scholar]
- Bothe H., Distler E., Eisbrenner G. Hydrogen metabolism in blue-green algae. Biochimie. 1978;60(3):277–289. doi: 10.1016/s0300-9084(78)80824-4. [DOI] [PubMed] [Google Scholar]
- Bothe H., Tennigkeit J., Eisbrenner G. The utilization of molecular hydrogen by the blue-green alga Anabaena cylindrica. Arch Microbiol. 1977 Jul 26;114(1):43–49. doi: 10.1007/BF00429628. [DOI] [PubMed] [Google Scholar]
- Carpenter E. J., Price C. C. Marine oscillatoria (Trichodesmium): explanation for aerobic nitrogen fixation without heterocysts. Science. 1976 Mar 26;191(4233):1278–1280. doi: 10.1126/science.1257749. [DOI] [PubMed] [Google Scholar]
- Chan Y. K., Nelson L. M., Knowles R. Hydrogen metabolism of Azospirillum brasilense in nitrogen-free medium. Can J Microbiol. 1980 Sep;26(9):1126–1131. doi: 10.1139/m80-186. [DOI] [PubMed] [Google Scholar]
- Donze M., Haveman J., Schiereck P. Absence of photosystem 2 in heterocysts of the blue-green alga Anabaena. Biochim Biophys Acta. 1972 Jan 21;256(1):157–161. doi: 10.1016/0005-2728(72)90170-3. [DOI] [PubMed] [Google Scholar]
- Fay P., Kulasooriya S. A. Tetrazolium reduction and nitrogenase activity in heterocystous blue-green algae. Arch Mikrobiol. 1972;87(4):341–352. doi: 10.1007/BF00409133. [DOI] [PubMed] [Google Scholar]
- Lim S. T. Determination of Hydrogenase in Free-living Cultures of Rhizobium japonicum and Energy Efficiency of Soybean Nodules. Plant Physiol. 1978 Oct;62(4):609–611. doi: 10.1104/pp.62.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKUI S., SUZUKI Y., MOMOSE K., OGAMO A. Chemical properties and enzymatic reduction of neotetrazolium chloride. J Biochem. 1963 Jun;53:500–502. doi: 10.1093/oxfordjournals.jbchem.a127729. [DOI] [PubMed] [Google Scholar]
- Peterson R. B., Burris R. H. Conversion of acetylene reduction rates to nitrogen fixation rates in natural populations of blue-green algae. Anal Biochem. 1976 Jun;73(2):404–410. doi: 10.1016/0003-2697(76)90187-1. [DOI] [PubMed] [Google Scholar]
- Peterson R. B., Wolk C. P. High recovery of nitrogenase activity and of Fe-labeled nitrogenase in heterocysts isolated from Anabaena variabilis. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6271–6275. doi: 10.1073/pnas.75.12.6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. B., Wolk C. P. Localization of an uptake hydrogenase in anabaena. Plant Physiol. 1978 Apr;61(4):688–691. doi: 10.1104/pp.61.4.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W. D., Fitzgerald G. P., Burris R. H. In situ studies on N2 fixation using the acetylene reduction technique. Proc Natl Acad Sci U S A. 1967 Nov;58(5):2071–2078. doi: 10.1073/pnas.58.5.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W. D., Haystead A., Pearson H. W. Nitrogenase activity in heterocysts of blue-green algae. Nature. 1969 Oct 18;224(5216):226–228. doi: 10.1038/224226a0. [DOI] [PubMed] [Google Scholar]
- Tel-Or E., Luijk L. W., Packer L. Hydrogenase in N2-fixing cyanobacteria. Arch Biochem Biophys. 1978 Jan 15;185(1):185–194. doi: 10.1016/0003-9861(78)90158-3. [DOI] [PubMed] [Google Scholar]
- Winkenbach F., Wolk C. P. Activities of enzymes of the oxidative and the reductive pentose phosphate pathways in heterocysts of a blue-green alga. Plant Physiol. 1973 Nov;52(5):480–483. doi: 10.1104/pp.52.5.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R., Iturriaga R., Becker-Birck J. Simultaneous determination of the total number of aquatic bacteria and the number thereof involved in respiration. Appl Environ Microbiol. 1978 Dec;36(6):926–935. doi: 10.1128/aem.36.6.926-935.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]