Abstract
Immunization with live attenuated simian immunodeficiency virus (SIV) strains has proved to be one of the most effective strategies to induce protective immunity in the SIV/macaque model. To better understand the role that CD4+ T helper responses may play in mediating protection in this model, we characterized SIV-specific proliferative and cytokine responses in macaques immunized with live attenuated SIV strains. Macaques chronically infected with live attenuated SIV had strong proliferative responses to SIV proteins, with stimulation indices of up to 74. The magnitude of the proliferative response to SIV Gag varied inversely with the degree of attenuation; Gag-specific but not envelope-specific responses were lower in animals infected with more highly attenuated SIV strains. SIV-specific stimulation of lymphocytes from vaccinated macaques resulted in secretion of interferon-γ, IL-2, regulated-upon-activation, normal T cells expressed and secreted (RANTES), macrophage inflammatory protein (MIP)-1α, and MIP-1β but not IL-4 or IL-10. Intracellular flow cytometric analysis documented that, in macaques vaccinated with SIVmac239Δnef, up to 2% of all CD4+T cells were specific for SIV p55. The ability of live attenuated SIV to induce a strong, sustained type 1 T helper response may play a role in the success of this vaccination approach to generate protection against challenge with wild-type SIV.
One of the significant barriers to the development of an effective AIDS vaccine has been the lack of understanding regarding what type of immune responses are able to protect against HIV infection. Studies in HIV-exposed but uninfected individuals and HIV-infected subjects with low viral loads and normal immune function have suggested that induction of both neutralizing antibody and cytotoxic T lymphocyte (CTL) responses may be associated with effective control of HIV (1, 2). However, it has been difficult to draw definitive conclusions from these studies, in part because of the lack of information as to whether these subjects are truly protected from HIV infection or disease. The impressive ability of live attenuated SIV vaccines to induce protection against challenge with pathogenic strains of SIV (3) has generated a valuable model in which to examine the potential mechanisms responsible for protection. A number of studies have demonstrated that animals vaccinated with live attenuated SIV resist intravenous and mucosal infection with pathogenic SIV strains (3–5). Animals vaccinated with live attenuated SIV develop neutralizing antibodies (3, 6), and a relatively vigorous CTL response (7–9) associated with the production of soluble antiviral factors, including the β-chemokines regulated-upon-activation, normal T cells expressed and secreted (RANTES), macrophage inflammatory protein (MIP)-1α, and MIP-1β (10). However, the relationship of these immune responses to protective immunity remains poorly understood.
CD4+ T helper cells play an important role in maintaining effective immunity against viral pathogens (11). In murine models, virus-specific T helper responses are important in maintaining effective CTL responses against viral pathogens such as lymphotropic murine herpesvirus-68 (12) and lymphocytic choriomeningitis virus (13). In the absence of CD4+ T cell responses, virus-specific CTL may either be eliminated or persist yet be nonfunctional (14). In HIV and SIV infection, virus-specific T helper responses as assessed by standard proliferation assays are generally low to absent (15, 16). However, recent studies have demonstrated that vigorous HIV-1-specific CD4+ T lymphocyte proliferative responses can be detected in HIV-1-infected long-term nonprogressors and in subjects treated with antiretroviral therapy early in the course of HIV infection (17). Defining the mechanisms resulting in the development or failure of T helper cell responses and their correlation with immune protection should help elucidate the immunopathogenesis of SIV and HIV-1 infection and may provide valuable insight into therapeutic interventions and vaccine development.
To better understand the role that CD4+ T helper responses may play in protective immunity in the SIV/macaque model, we characterized SIV-specific proliferative and cytokine responses in rhesus macaques immunized with live attenuated SIV strains. Using both standard proliferation assays and new techniques that allow visualization of CD4+ T helper cells on a single-cell basis, we now demonstrate that vaccination with live attenuated SIV results in the induction of a vigorous and sustained type 1 T helper response.
Materials and Methods
Animals.
Rhesus macaques used for this study were infected for 3 to 9 years with the following live attenuated SIV strains: SIVmac239Δnef, which contains a single 182-bp deletion in nef (18); SIVmac239Δ3 [deficient in nef, vpr, and the upstream sequences (US) of the long terminal repeat (6)]; SIVmac239Δ3x (deficient in nef, vpx, and US sequences); or SIVmac239Δ4 (deficient in nef, vpr, vpx, and US) (19). A subset of these animals had been challenged 1 to 7 years before our study either with cloned pathogenic SIVmac239 or uncloned pathogenic SIVmac251. All challenged macaques remained healthy without evidence for wild-type SIV infection at the time of our study (3, 5, 6), and no animal received any booster immunizations at any time. A second group of rhesus macaques used for this study included animals infected with the pathogenic strains SIVmac239 or SIVmac251. At the time of the study, macaques infected with pathogenic SIV strains had relatively advanced disease, with CD4 counts = 300 mm3. Uninfected normal macaques from our conventional colony served as negative controls. All rhesus macaques were maintained in accordance with the guidelines of the local institutional animal-use committees and the National Institutes of Health (39).
Preparation of Rhesus Macaque Peripheral Blood Mononuclear Cells (PBMCs).
Rhesus macaque PBMCs were isolated from fresh heparinized blood by density gradient centrifugation (Ficoll 1077, Sigma) and resuspended in RPMI medium 1640 (Sigma) supplemented with 10% non-heat-inactivated fetal calf serum (Sigma), 10 mM Hepes, 2 mM l-glutamine, 50 units of penicillin per ml, and 50 μg of streptomycin per ml (R-10 medium). Where indicated, PBMCs were separated into CD8+ and CD4+ T lymphocyte subsets by negative selection with immunomagnetic beads (Dynabeads; Dynal, Oslo, Norway) as described previously (10).
Proliferation Assays.
Whole PBMCs or fractionated cell populations were cultured in triplicate or quadruplicate wells at 1 × 105 cells per well in a total volume of 200 μl of R-10 medium in the presence of SIV recombinant proteins, control protein, or medium alone for 6 days. The SIV p55 protein (20) and Mr 160,000 glycoprotein (gp160) (21) were derived from SIVmac239 and produced in a baculovirus expression system as previously described. The vesicular stomatitis virus-nucleocapsid (VSV-N) protein, also produced by using a baculovirus expression system, was used as a control antigen (22). Unless otherwise indicated, SIV p55 was added at concentrations ranging from 5 to 2.5 μg/ml, whereas gp160 was added at a concentration of 1 μg/ml. 3H-labeled thymidine (1.0 μCi) was then added to each well and harvested after 18 h with an automated cell harvester (TOMTEC; Wallac, Turku, Finland). 3H-labeled thymidine incorporation was determined with a Microβeta Plus liquid scintillation counter (Wallac) and results were expressed as counts per minute (cpm), or as a stimulation index (S.I.), defined as the ratio of the mean cpm of wells incubated with SIV antigen per mean CPM of wells incubated with medium alone. Controls included VSV-N at concentrations ranging from 5 to 2.5 μg/ml and concanavalin A (ConA, Sigma) at 5 μg/ml. Based on 10 assays with six uninfected controls, an S.I. ≥ 3 was considered positive.
Immunofluorescent Staining and Flow Cytometric Analysis.
Three- or four-color immunophenotyping was performed by using mAbs specific for CD3, CD4, and CD8. mAbs used for flow cytometric analysis were conjugated with fluorescein isothiocyanate, phycoerythrin, allophycocyanin, or peridinin chlorophyl protein. These antibodies included CD4 (Leu-3a) and CD8 (Leu-2a), which were obtained from Becton-Dickinson Immunocytometry Systems, and CD3 (6G12), which was kindly provided by Johnson Wong (Massachusetts General Hospital, Boston, MA) (23). Briefly, after antigen-specific stimulation, 0.5 × 106 PBMCs were washed with PBS containing 2% fetal calf serum and incubated with conjugated antibodies for 30 min at 4°C. After staining, the cells were washed, resuspended in 2% paraformaldehyde, and analyzed on a FACScan or FACSCalibur (Becton Dickinson).
Detection of intracellular cytokines in PBMCs was assessed by multiparameric flow cytometric analysis with antibodies previously shown to cross-react with rhesus cytokines (24). These antibodies included anti-IL-2 (clone MQ1–17H12), anti-IL-4 (clone 8DA-8), anti-IL-10 (clone JES3–9D7), anti-interferon-γ [(IFN-γ) clone 4S.B3)], mouse anti-human RANTES, and mouse anti-human MIP-1α. Control antibodies included mouse and rat Ig isotypes (IgG1 and IgG2a) (Becton Dickinson). To optimize stimulation of macaque PBMCs with processed SIV antigen, autologous adherent cells were prepared by plating 5 × 106 to 10 × 106 PBMCs per well of a 24-well plate. Nonadherent cells were washed away after 1 to 2 h and kept at 37°C overnight in R-10 medium. The remaining cells were pulsed overnight with SIV p55 or control antigen VSV-N (both at a final concentration of 2.5 μg/ml) and then incubated with 2 × 106 peripheral blood lymphocytes (PBL) ml−1 per well. After 24 h, cells were incubated for 4 h in the presence of 2 μM monensin (Sigma). Intracellular cytokine and β-chemokine analyses were then performed, and expression was determined in T lymphocyte subsets as previously described (24). For each analysis, a minimum of 20,000 events was acquired. Lymphocytes were gated on forward and side scatter, and the proportion of lymphocytes expressing the cytokine or chemokine of interest was determined using CELLQuest (Becton Dickinson). Gates for the determination of cytokine-staining cells were determined so as to yield less than 0.5% cytokine-expressing cells after stimulation with the control antigen VSV-N.
Cytokine and β-Chemokine Production.
Production of the cytokines IFN-γ, IL-2, IL-4, and IL-10 and the β-chemokines RANTES, MIP-1α, and MIP-1β was determined by ELISA (IFN-γ, Cytoscreen Immunoassay, BioSource International, Camarillo, CA; IL-2, Genzyme; IL-4 and IL-10, Immunotech, Westbrook, ME; RANTES, MIP-1 α, and MIP-1-β, R&D Systems). Briefly, 1 × 106 PBMCs in 1 ml of R-10 medium were incubated in individual wells of a 24-well plate with SIV p55 at 2.5 μg/ml. Supernatants were collected from days 1 to 6 and analyzed for cytokine and β-chemokine production by ELISA as recommended by the manufacturer.
Enzyme-Linked Immunospot (ELISPOT) Assays for IFN-γ-Secreting Cells.
ELISPOT assays for IFN-γ-secreting cells were performed with a commercial kit (U-Cytech bu, Utrecht, The Netherlands), based on a previously published technique adapted for rhesus macaques (25). Briefly, 96-well microtiter U-bottom plates (Greiner, Nurtingen, Germany) were coated overnight at 4°C with 100 μl per well of a murine mAb (MD1) specific for human IFN-γ that also crossreacts well with rhesus IFN-γ (25). CD4+ T cells were stimulated with autologous adherent cells pulsed with SIV p55 or VSV-N, as described above, for 24 h and then added at 2.5 × 105, 5 × 105, and 10 × 105 cells per well. After incubation, the plates were washed followed by incubation with a polyclonal, biotinylated anti-IFN-γ antibody (25). Spot-forming cells were then identified by using an alkaline-phosphatase anti-biotin antibody, followed by incubation with a silver-containing substrate and enumeration of spots with an inverted microscope. The number of p55-specific cells was calculated by subtracting the number of IFN-γ-producing cells after stimulation with VSV-N from the number of spots observed after stimulation with SIV p55.
Statistical Analysis.
Linear regressions, ANOVA, and Mann-Whitney tests were performed by using StatView (StatView Reference, Abacus Concepts, Berkeley, CA)
Results
Detection of Vigorous SIV-Specific T Cell-Proliferative Responses in Macaques Immunized with Live Attenuated SIV.
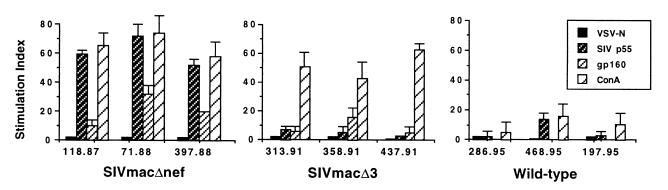
We initially analyzed SIV-specific proliferative responses in rhesus macaques chronically infected with SIVmac239Δnef or SIVmac239Δ3 and compared these responses with those from uninfected normal animals and animals infected with wild-type SIV strains. Proliferation assays were performed 4 to 6 times on each animal with highly reproducible results. As shown in Fig. 1, the overall stimulation of PBMCs with SIV p55 resulted in vigorous antigen-specific proliferative responses in three SIVmac239Δnef-immunized macaques, with S.I.s up to 74. The range of stimulation indices in these three SIVmac239Δnef-immunized animals was 53 to 74 (mean S.I., 63) with net Δcpm ranging from 10,448 to 18,160 (mean Δcpm = 14,304). Macaques vaccinated with SIVmac239Δ3 also had significant proliferative responses to SIV p55, although lower than SIVmac239Δnef-vaccinated animals, with S.I.s up to 8. Strong proliferative responses to gp160 were also detected in all three macaques immunized with SIVmac239Δnef or SIVmac239Δ3. In contrast, macaques infected with pathogenic SIV had undetectable- to low-level proliferative responses to SIV p55 or gp160 (S.I.s < 4), except for one macaque (animal 468.95) that had detectable responses to SIV p55 (S.I. = 14). Proliferative responses to ConA were also significantly suppressed in SIV-infected animals. Proliferative responses to SIV p55 or gp160 in six uninfected controls yielded S.I.s of <3 (data not shown). Fractionation of cells by using immunomagnetic beads demonstrated that SIV p55-specific proliferative responses were mediated by CD4+ T cells, and inhibition assays with mAbs demonstrated that these responses were class II MHC-restricted (26).
Figure 1.
Detection of strong SIV-specific proliferative responses in macaques immunized with live attenuated SIV. Controls included the negative control antigen VSV-N and the positive control ConA.
SIV p55-Specific Proliferative Responses in Macaques Immunized with Live Attenuated SIV Vaccine Vary Inversely with the Degree of Attenuation.
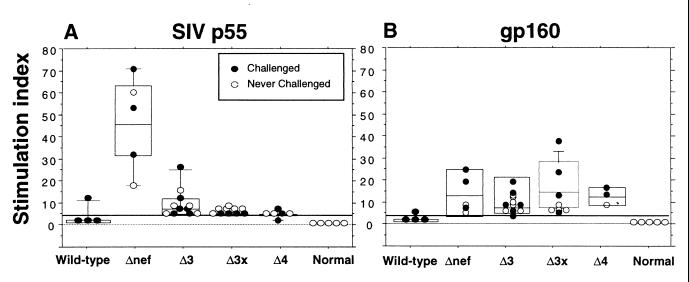
Based on the apparent decreased proliferative responses to SIV p55 in macaques infected with SIVmac239Δ3 as compared with SIVmac239Δnef, we examined the relationship between SIV-specific proliferative responses and the degree of attenuation of the infecting virus in a larger cohort of vaccinated animals. Several different groups of SIV-infected macaques were studied, including animals immunized with the live attenuated SIV strains SIVmac239Δnef (n = 5), SIVmac239Δ3 (n = 11), SIVmac239Δ3x (n = 10), or SIVmac239Δ4 (n = 6) or animals infected with pathogenic SIV strains (n = 4) and uninfected normal animals (n = 5). These strains, listed in increasing order of attenuation, varied by over 100-fold in in vivo replicative capacity (19). SIV p55-specific proliferative responses were significantly greater in animals infected with SIVmac239Δnef as compared with animals infected with more attenuated SIV strains or wild-type SIV-infected animals (ANOVA, P < 0.002 for all comparisons) (Fig. 2A). It is interesting that we did not observe a significant effect between SIV attenuation and proliferative responses to gp160 (Fig. 2B). Although several vaccinated animals had been previously challenged with pathogenic SIV and found to be uninfected, no differences in SIV-specific proliferative responses were observed between unchallenged and challenged-vaccinated animals.
Figure 2.
Comparative analysis of T cell-mediated proliferative responses in macaques immunized with live attenuated SIV strains. Data from a total of 22 vaccinated animals are shown, including data from animals presented in Fig. 1. Horizontal bars show median values, boxes indicate 75% confidence intervals, and error bars show 95% confidence intervals. The solid horizontal lines indicate the lower limit for significant SIV-specific stimulation indices. p55-specific proliferative responses in SIVΔnef-immunized animals are significantly greater compared with responses in animals infected with more attenuated SIV strains (P < 0.002, ANOVA).
SIV-Specific Stimulation of T Lymphocytes from Macaques Immunized with Live Attenuated SIV Induces Secretion of IFN-γ and IL-2, but Not IL-4 or IL-10.
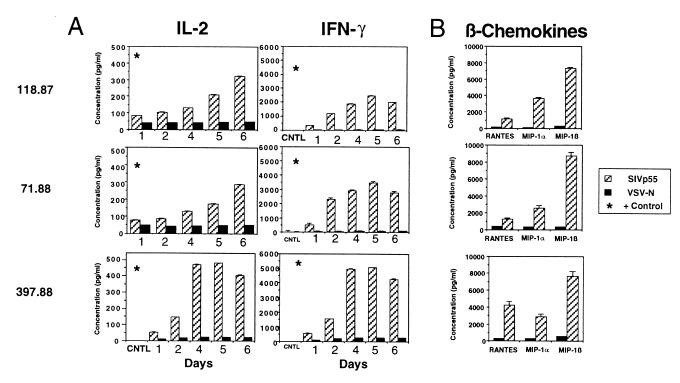
To assess the range of cytokines produced by SIV-specific CD4+ T cells in vaccinated macaques, we investigated the production of IFN-γ, IL-2, IL-4, and IL-10 after stimulation of PBMCs with SIV p55. Using PBMCs from SIVmac239Δnef-immunized macaques, we observed specific release of IFN-γ ranging from 2500 pg/ml to 4120 pg/ml (Fig. 3A). Stimulation with the control antigen VSV-N induced only up to 200 pg/ml of IFN-γ from vaccinated animals, and no significant release of IFN-γ (<55 pg/ml) was observed after stimulation of PBMCs from normal animals with p55 (data not shown). Similarly, IL-2 production from PBMCs from immunized animals was increased by five- to sixfold after stimulation with SIV p55 as compared with control antigen. However, SIV p55 stimulation did not result in specific production of IL-4 (<10–30 pg/ml) or IL-10 expression (<35 pg/ml, data not shown). Stimulation with PMA/Ca2+ ionophore or ConA led to release of up to 463 pg/ml of IL-4 and 439 pg/ml of IL-10 (data not shown). Immunization with live attenuated SIV thus induces a type 1 T helper response, characterized by production of IL-2 and IFN-γ, but not IL-4 or IL-10.
Figure 3.
Production of cytokines and β-chemokines in PBMC from SIVmacΔnef-vaccinated animals after antigen-specific stimulation. (A) Production of IFN-γ and IL-2 after SIVp55-specific stimulation of PBMCs from three SIVmacΔnef-immunized animals. Positive controls included tetradecanoylphorbol 13 acetate/calcium ionophore or ConA-stimulated PBMCs (*). (B) Production of β-chemokines RANTES, MIP-1α, and MIP-1β after SIV p55-specific stimulation.
β-Chemokine Secretion by SIV p55-Stimulated T Lymphocytes from SIVmac239Δnef-Immunized Macaques.
Chemokines are secreted by both CD4+ and CD8+ T cells during the generation of an immune response. The β-chemokines RANTES, MIP-1α, and MIP-1β are able to inhibit replication of both HIV (27) and SIV (10, 28). In addition, release of RANTES has been reported to enhance the function of HIV-specific CTL (29). We therefore evaluated whether T lymphocytes from macaques immunized with live attenuated SIVmac239Δnef were able to produce these β-chemokines after SIV p55 stimulation (Fig. 3B). Incubation of PBMCs isolated from SIVmac239Δnef-immunized animals with SIV p55 induced secretion of 1 to 8 ng/ml RANTES, MIP-1α, and MIP-1β, levels that were 8- to 15-fold greater than those observed by using PBMCs from an uninfected control. No significant increase of β-chemokines was observed after stimulation with control antigen in either immunized animals or controls. These results suggest that secretion of RANTES, MIP-1α, and MIP-1β may be another mechanism by which induction of virus-specific CD4+ T helper responses may directly suppress SIV replication in macaques.
ELISPOT Analysis of SIV p55-Specific CD4+T Cells in Macaques Immunized with Live Attenuated SIV.
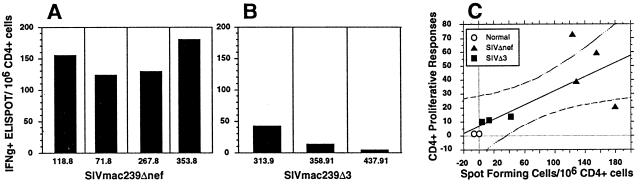
Although lymphocyte proliferation assays have traditionally been used to evaluate antigen-specific T helper responses, these assays have a number of limitations, including difficulties with precise quantitation of responses. The observation that stimulation with SIV p55 led to relatively vigorous production of IFN-γ allowed us to use recently developed techniques that include detection of IFN-γ-secreting cells as an alternative approach to examine SIV-specific CD4 responses. Freshly isolated CD4+ T cells from SIVmac239Δnef (n = 4)- and SIVmac239Δ3 (n = 3)-immunized macaques were stimulated with SIV p55 and then antigen-specific cells were identified by ELISPOT analysis with antibodies specific for IFN-γ. As previously observed for proliferative responses, animals immunized with SIVmac239Δnef had higher levels of spot-forming CD4+ T cells than did animals infected with SIVmac239Δ3 (mean ± SD of 147 ± 26 vs. 15 ± 10 cells per 106 cells; P < 0.001, Mann–Whitney U test) (Fig. 4 A and B). In uninfected macaques (n = 2), less than 10 spots per 106 CD4+ T cells were observed after stimulation with p55 (data not shown). In general, the frequency of SIV p55-specific CD4+ T cells as assessed by ELISPOT correlated well with the bulk proliferative responses (r = 0.7) (Fig. 4C).
Figure 4.
ELISPOT analysis of SIV-specific CD4+ T cells from vaccinated macaques. CD4+ T cells from animals immunized with SIVmac239nef (A) or SIVmac239Δ3 (B) were stimulated with autologous antigen-presenting cells pulsed with SIV p55 and IFN-γ-producing cells identified by using ELISPOT analysis. (C) Correlation of SIV p55-CD4+ T cells as assessed by ELISPOT with proliferative responses by using PBMCs isolated from the Δnef- or Δ3-immunized macaques.
Intracellular Cytokine Analysis of SIV p55-Specific CD4+ T Cells.
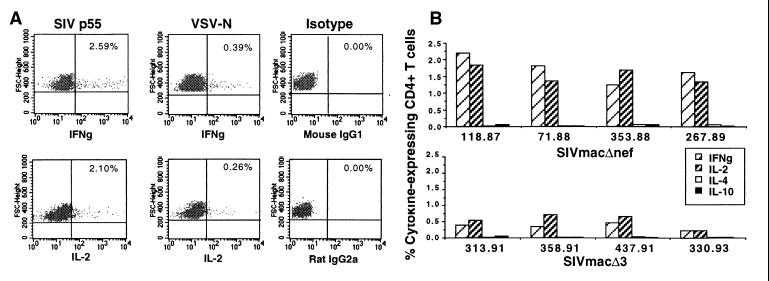
The use of flow cytometric techniques that allow for the identification of cytokine-secreting cells on a single-cell basis has permitted precise quantitation of the frequency of cytokine-secreting cells (30). We examined expression of intracellular cytokines and β-chemokines in CD4+ T cells after stimulation with autologous antigen-presenting cells pulsed with SIV p55. Initial studies in a macaque immunized with SIVmac239Δnef demonstrated a relatively high frequency of SIV p55-specific CD4+ T cells after 24 h of stimulation, representing 2.2% and 1.8% of all CD4+ T cells for IFN-γ and IL-2, respectively (Fig. 5A). Examination of antigen-specific responses in multiple animals vaccinated with SIVmac239Δnef demonstrated similar frequencies of IFN-γ- (1.72 ± 0.4%) and IL-2- (1.52 ± 0.2%) secreting cells but no significant production of IL-4 and IL-10 expression (<0.1% for both; Fig. 5B and data not shown). As assessed by analysis of either IFN-γ or IL-2, the frequency of SIV p55-specific CD4+ T cells was significantly greater in animals immunized with SIVmac239Δnef as compared with animals immunized with more attenuated SIV strains (P < 0.001, ANOVA). No IL-2, IL-4, IL-10, or IFN-γ secretion above background (>0.25% p55-specific staining) was detected after p55 stimulation of PBMCs from wild-type infected animals or uninfected normal controls (data not shown). Although levels of expression of the β-chemokines RANTES, and MIP-1α after 24 h of stimulation with p55 were not above background, analysis of expression after 4 to 6 days’ stimulation with SIV p55 demonstrated frequencies of chemokine-expressing CD4+ T cells of 4.5% to 2.5%, respectively (data not shown).
Figure 5.
Intracellular cytokine analysis of CD4+ T cells after stimulation with SIV p55. (A) PBMCs from an SIVmac239Δnef-immunized macaque were stimulated with SIV p55- or VSV-N-pulsed autologous adherent cells for 24 h, and then intracellular expression of IL-2 and IFN-γ in CD4+ T lymphocytes was evaluated by flow cytometry. The numbers shown in the upper right-hand corner of each panel in A correspond to the percentages of CD4+ T cells expressing the given cytokine. (B) Intracellular cytokine expression by SIV p55-specific CD4+ T cells from macaques infected with live attenuated SIV strains.
Discussion
Despite extensive study, the specific immune responses responsible for protection against HIV and SIV infection remain poorly understood. In this study, we characterized SIV-specific CD4+ T helper responses in rhesus macaques immunized with live attenuated SIV strains, one of the most effective vaccine approaches studied in nonhuman primates (31). We now show that macaques chronically infected with live attenuated virus develop relatively strong and persistent type 1 T helper response associated with secretion of β-chemokines. These virus-specific CD4+ T cells were found at a relatively high frequency in vaccinated macaques, as demonstrated by flow cytometric analysis, which revealed that up to 2.0% of all CD4+ T cells in vaccinated macaques were SIV-specific.
The magnitude and characteristics of the virus-specific CD4+ T cell response in macaques vaccinated with live attenuated SIV strains are similar in many respects to the CD4+ T cell responses recently reported in HIV-infected subjects with control of viremia (17). Long-term nonprogressing HIV-1-infected subjects or subjects who had received antiretroviral therapy early in the course of infection had responses to viral antigens in bulk proliferation assays that were comparable to those we observed in SIVmac239Δnef-vaccinated macaques. Using limiting dilution analysis proliferation assays, we have observed a frequency of up to 96 SIV p55-specific precursors per 106 cells for SIVmacΔnef-immunized macaques (26), similar to the frequency of HIV-1 proliferative cells reported by Rosenberg et al. (17). Due to technical limitations inherent in limiting dilution assays, these values are quite likely to represent a significant underestimate of the true frequency of virus-specific T cells (32). In both our study and the study by Rosenberg et al. (17), virus-specific stimulation resulted in production of IFN-γ, IL-2, and the β-chemokines RANTES and MIP-1α. Taken together, these studies suggest that containment of HIV or SIV replication by any one of several mechanisms allows the development of virus-specific CD4+ T helper responses.
Assessment of HIV or SIV-specific T helper responses with standard proliferation assays in subjects infected with pathogenic viruses has generally revealed low to absent responses (15, 16). The failure to develop strong virus-specific CD4+ T helper responses during pathogenic HIV or SIV infection has been postulated to be related to the preferential replication of these primate lentiviruses in activated CD4+ T cells and the ability of infected antigen-presenting cells to transmit virus to responding CD4+ T cells (33). However, a recent report that used intracellular cytokine analysis of antigen-stimulated cells demonstrated relatively high levels of HIV-specific CD4+ T cells even in subjects with relatively advanced disease (30). The discrepancies between results obtained with these different techniques may in part reflect the fact that proliferation assays require culturing of cells for periods of 3 to 6 days, during which time cells may undergo apoptosis. However, our results show a general concordance between a variety of different techniques used to assess CD4+ T lymphocyte function in macaques vaccinated with attenuated SIV strains, including proliferation assays, ELISPOT analysis, and intracellular cytokine staining. Taken together, all of these techniques demonstrate a relatively high frequency of SIV-specific CD4+ T cells in macaques immunized with SIVmac239Δnef and an inverse relationship between Gag-specific responses and the degree of attenuation of the immunizing strain. In addition, our observations also suggest the possibility that one cause of relatively vigorous HIV-1-specific proliferative responses in long-term nonprogressors may be infection with attenuated HIV-1 strains, as has been reported for subjects infected with naturally occurring variants deficient in nef (34).
An interesting and unexpected finding from these studies was the observation that proliferative responses to Gag but not envelope correlated inversely with the degree of virus attenuation. In fact, relatively strong proliferative responses (S.I.s of 8–20) to envelope were observed even in animals infected with the highly attenuated strain SIVmac239Δ4. The mechanism responsible for the difference in proliferative responses to Gag and envelope is not known, but may be related to differences in presentation of these antigens, in particular, the ability of gp120 to bind directly to and be processed by CD4+ T cells (35).
A variety of observations suggests that induction of virus-specific CD4+ T helper responses may be an important characteristic of an effective AIDS vaccine. In several murine models of chronic viral infection, CD4+ T cell responses are required to maintain an effective CTL response and to achieve sustained control of viral replication (12, 13). In mice immunized with an attenuated murine retrovirus, adoptive transfer of both CD4+ and CD8+ T cells is required for effective protection against a pathogenic retrovirus (36). Similarly, in a recent vaccine study with recombinant subunit vaccination (37), protection against challenge with a chimeric simian/HIV correlated with induction of a strong and persistent T helper response, as well as with the titer of neutralizing antibodies and production of β-chemokines by CD8+ T cells. SIV-specific proliferative responses have also been reported in macaques infected with a different nef-defective strain, SIVmacpC8, which was also proved effective in inducing protection against challenge with pathogenic SIV strains (38). SIV-specific T helper responses might contribute to protective immunity via several mechanisms including enhancement of SIV-specific CTL responses, either by release of RANTES (29) or other cytokines such as IL-2, direct antiviral effects of the release of β-chemokines, or enhancement of immune responses other than CTL.
We have provided evidence that live attenuated SIV vaccines induce strong, sustained type 1 CD4+ T helper responses that may play an important role in the success of this vaccination approach to generate protection against challenge with wild-type SIV. A better characterization of immune responses induced by live attenuated SIV vaccines may yield clues to the nature of protective immunity against HIV.
Acknowledgments
We thank Ron Desrosiers, Kelledy Manson and Michael Wyand for providing samples from SIV-infected animals; Ron Desrosiers, Eric Rosenberg, and Bruce Walker for helpful discussions and review of the manuscript; Peter van der Miede and Amitinder Kaur for technical advice; and Michael Rosenzweig and MaryAnn DeMaria for assistance with flow cytometry. This work was supported by Public Health Service Grants RR 00168, AI43044, AI45314 (R.P.J.), and AI36197 (T.Y.) and Department of Army Grant DAMD17-95-C-5054 (T.Y.). M-C.G. is supported by a Pediatric AIDS Foundation Scholar Award. R.P.J. is an Elizabeth Glaser Scientist and is supported by the Elizabeth Glaser Pediatric AIDS Foundation.
Abbreviations
- SIV
simian immunodeficiency virus
- PBMC
peripheral blood mononuclear cell
- S.I.
stimulation index
- RANTES
regulated-upon-activation, normal T cells expressed and secreted
- MIP
macrophage inflammatory protein
- ELISPOT
enzyme-linked immunospot
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Rowland-Jones S, Sutton J, Ariyoshi K, Dong T, Gotch F, McAdam S, Whitby D, Sabally S, Gallimore A, Corrah T, et al. Nat Med. 1995;1:59–64. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- 2.Pantaleo G, Fauci A S. Annu Rev Immunol. 1995;13:487–512. doi: 10.1146/annurev.iy.13.040195.002415. [DOI] [PubMed] [Google Scholar]
- 3.Daniel M D, Kirchhoff F, Czajak S C, Sehgal P K, Desrosiers R C. Science. 1992;258:1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- 4.Cranage M, Whatmore A, Sharpe S, Cook N, Polyanskaya N, Leech S, Smith J, Rud E, Dennis M, Hall G. Virology. 1997;229:143–54. doi: 10.1006/viro.1996.8419. [DOI] [PubMed] [Google Scholar]
- 5.Johnson R, Lifson J, Czajak S, Cole K, Manson K, Glickman R, Yang J, Montefiori D, Montelaro R, Wyand M, Desrosiers R. J Virol. 1999;73:4952–4961. doi: 10.1128/jvi.73.6.4952-4961.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wyand M S, Manson K H, Garcia-Moll M, Montefiori D, Desrosiers R C. J Virol. 1996;70:3724–3733. doi: 10.1128/jvi.70.6.3724-3733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dittmer U, Nisslein T, Bodemer W, Petry H, Sauermann U, Stahl H C, Hunsmann G. Virology. 1995;212:392–397. doi: 10.1006/viro.1995.1496. [DOI] [PubMed] [Google Scholar]
- 8.Johnson R P, Glickman R L, Yang J Q, Kaur A, Dion J T, Mulligan M J, Desrosiers R C. J Virol. 1997;71:7711–7718. doi: 10.1128/jvi.71.10.7711-7718.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu X-N, Screaton G R, Gotch F M, Dong T, Tan R, Almond N, Walker B, Stebbings R, Kent K, Nagata S, Stott J E, McMichael A J. J Exp Med. 1997;186:7–16. doi: 10.1084/jem.186.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gauduin M-C, Glickman R L, Means R, Johnson R P. J Virol. 1998;72:6315–6324. doi: 10.1128/jvi.72.8.6315-6324.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalams S, Walker B. J Exp Med. 1998;188:2199–2204. doi: 10.1084/jem.188.12.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardin R D, Brooks J W, Sarawar S R, Doherty P C. J Exp Med. 1996;184:863–871. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matloubian M, Conception R J, Ahmed R. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zajac A, Blattman J, Murali-Krishna K, Sourdive D, Suresh M, Altman J, Ahmed R. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dittmer U, Luke W, Stahl-Henning C, Coulibaly C, Petry H, Bodemer W, Hunamann G, Voss G. J Med Primatol. 1994;23:298–303. doi: 10.1111/j.1600-0684.1994.tb00287.x. [DOI] [PubMed] [Google Scholar]
- 16.Wahren B, Morfeldt-Månsson L, Biberfeld G, Moberg L, Sönnerborg A, Ljungman P, Werner A, Kurth R, Gallo R, Bolognesi D. J Virol. 1987;61:2017–2023. doi: 10.1128/jvi.61.6.2017-2023.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenberg E, Billingsley J, Caliendo A, Boswell S, Sax P, Kalams S, Walker B. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 18.Kestler H W, Ringler D J, Mori K, Panicali D L, Sehgal P D, Daniel M D, Desrosiers R C. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 19.Desrosiers R, Lifson J, Gibson J, Csajak S, Howe A, Arthur L. J Virol. 1998;72:1431–1437. doi: 10.1128/jvi.72.2.1431-1437.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giavedoni L, Ahmad S, Jones L, Yilma T. J Virol. 1997;71:866–872. doi: 10.1128/jvi.71.2.866-872.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmad S, Lohman B, Marthas M, Giavedoni L, El-Amad Z, Haigwood N, Scandella C, Gardner M, Luciw P, Yilma T. AIDS Res Hum Retroviruses. 1994;10:195–204. doi: 10.1089/aid.1994.10.195. [DOI] [PubMed] [Google Scholar]
- 22.Ahmad S, Bassiri M, Banerjee A K, Yilma T. Virology. 1993;192:207–216. doi: 10.1006/viro.1993.1023. [DOI] [PubMed] [Google Scholar]
- 23.Kawai T, Wong J, MacLean J, Cosimi A B, Wee S. Transplant Proc. 1994;26:1845–1846. [PubMed] [Google Scholar]
- 24.Kaur A, Grant R, Means R, McClure H, Feinberg M, Johnson R. J Virol. 1998;75:9597–9611. doi: 10.1128/jvi.72.12.9597-9611.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van der Meide P, Groenestein R, de Labie M, Heeney P, Pala P, Slaoui M. J Med Primatol. 1995;24:271–281. doi: 10.1111/j.1600-0684.1995.tb00181.x. [DOI] [PubMed] [Google Scholar]
- 26.Gauduin, M.-C., Glickman, R., Ahmad, S., Yilma, T. & Johnson, R. (1999) J. Med. Prim., in press. [DOI] [PubMed]
- 27.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Tao L, Mitchell E, Bogers W, Doyle C, Bravery C, Bergmeier L, Kelly C, Heeney J, Lehner T. Proc Natl Acad Sci USA. 1998;95:5223–5228. doi: 10.1073/pnas.95.9.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hadida F, Vieillard V, Autran B, Clark-Lewis I, Baggioloni M, Debre P. J Exp Med. 1998;188:609–614. doi: 10.1084/jem.188.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pitcher C, Quittner C, Peterson D, Connors M, Koup R, Maino V, Picker L. Nat Med. 1999;5:518–525. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 31.Schultz A M, Stott E J. AIDS. 1994;8:S203–S212. [Google Scholar]
- 32.McMichael A, O’Callaghan C. J Exp Med. 1998;187:1367–1371. doi: 10.1084/jem.187.9.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Margolick J B, Volkman D J, Folks T M, Fauci A S. J Immunol. 1987;138:1719–1723. [PubMed] [Google Scholar]
- 34.Dyer W, Geczy A, Kent S, McIntyre L, Blasdall S, Learmont J, Sullivan J. AIDS. 1997;11:1565–1574. doi: 10.1097/00002030-199713000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Lanzavecchia A, Roosnek E, Gregory T, Berman P, Abrignani S. Nature. 1988;334:530–532. doi: 10.1038/334530a0. [DOI] [PubMed] [Google Scholar]
- 36.Dittmer U, Brooks D M, Hasenkrug K J. Nat Med. 1999;5:189–193. doi: 10.1038/5550. [DOI] [PubMed] [Google Scholar]
- 37.Heeney J, Teeuwsen V, van Gils M, Bogers W, De Giuli Morghen C, Radaelli A, Barnett S, Morein B, Akerblom L, Wang Y, et al. Proc Natl Acad Sci USA. 1998;95:10803–10808. doi: 10.1073/pnas.95.18.10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dittmer U, Brooks D, Hazenkrug K. J Virol. 1998;72:6554–6558. doi: 10.1128/jvi.72.8.6554-6558.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Committee on Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. Bethesda: Natl. Inst. Health; 1985. , DHHS Publ. No. (NIH) 85-23. [Google Scholar]