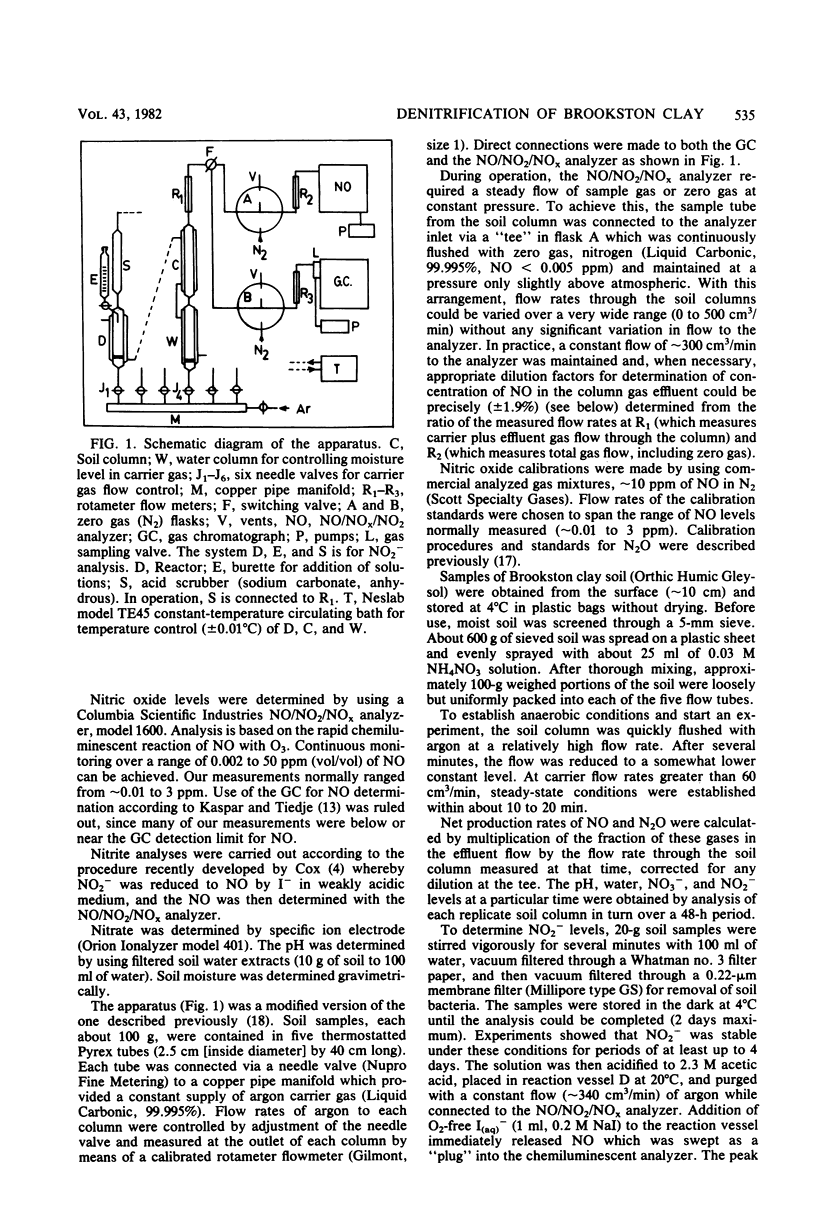
Abstract
Nitric oxide, nitrous oxide, and nitrite ion production was measured in a Brookston clay column undergoing anaerobic denitrification. A flow system method was used whereby argon carrier gas continuously stripped soil gases from the column, allowing steady-state rates to be obtained. Over several days the temporal change in rates of these gases and NO2− followed a pattern of increase and decay which may be expected of a reaction proceeding by several consecutive steps. The method permits observation of the relatively large net production rate of NO, which is normally not observed in static systems based on head space analysis of gaseous denitrification products. In the first several hours after the onset of anoxic conditions, the net rate of NO production, fNO, increased sharply to a maximum (∼1 × 10−10 mol of N/g of soil per min), paralleling the rapid increase in NO2− level, and then followed a more gradual decline extending over approximately 45 h. A similar but less pronounced pattern was observed for N2O, with net rates of production being considerably less than for NO. The ratio [NO-N]/[N2O-N] decreased with time from ∼2.5 at 6 h to ∼2.0 at 45 h. Estimated rates of N2 production appeared to be initially high, decreased rapidly within a few hours, and then gradually increased with time after the establishment of anaerobic conditions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Delwiche C. C., Bryan B. A. Denitrification. Annu Rev Microbiol. 1976;30:241–262. doi: 10.1146/annurev.mi.30.100176.001325. [DOI] [PubMed] [Google Scholar]
- Firestone M. K., Firestone R. B., Tiedje J. M. Nitric oxide as an intermediate in denitrification: evidence from nitrogen-13 isotope exchange. Biochem Biophys Res Commun. 1979 Nov 14;91(1):10–16. doi: 10.1016/0006-291x(79)90575-8. [DOI] [PubMed] [Google Scholar]
- Firestone M. K., Tiedje J. M. Temporal change in nitrous oxide and dinitrogen from denitrification following onset of anaerobiosis. Appl Environ Microbiol. 1979 Oct;38(4):673–679. doi: 10.1128/aem.38.4.673-679.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gersberg R., Krohn K., Peek N., Goldman C. R. Denitrification Studies with 13N-Labeled Nitrate. Science. 1976 Jun 18;192(4245):1229–1231. doi: 10.1126/science.192.4245.1229. [DOI] [PubMed] [Google Scholar]
- Goreau T. J., Kaplan W. A., Wofsy S. C., McElroy M. B., Valois F. W., Watson S. W. Production of NO(2) and N(2)O by Nitrifying Bacteria at Reduced Concentrations of Oxygen. Appl Environ Microbiol. 1980 Sep;40(3):526–532. doi: 10.1128/aem.40.3.526-532.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollocher T. C., Garber E., Cooper A. J., Reiman R. E. 13N,15N isotope and kinetic evidence against hyponitrite as an intermediate in dentrification. J Biol Chem. 1980 Jun 10;255(11):5027–5030. [PubMed] [Google Scholar]
- Payne W. J. Reduction of nitrogenous oxides by microorganisms. Bacteriol Rev. 1973 Dec;37(4):409–452. doi: 10.1128/br.37.4.409-452.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmer R. J., Kok B. Rate-temperature curves as an unambiguous indicator of biological activity in soil. Appl Environ Microbiol. 1979 Aug;38(2):224–228. doi: 10.1128/aem.38.2.224-228.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John R. T., Hollocher T. C. Nitrogen 15 tracer studies on the pathway of denitrification in Pseudomonas aeruginosa. J Biol Chem. 1977 Jan 10;252(1):212–218. [PubMed] [Google Scholar]


