Abstract
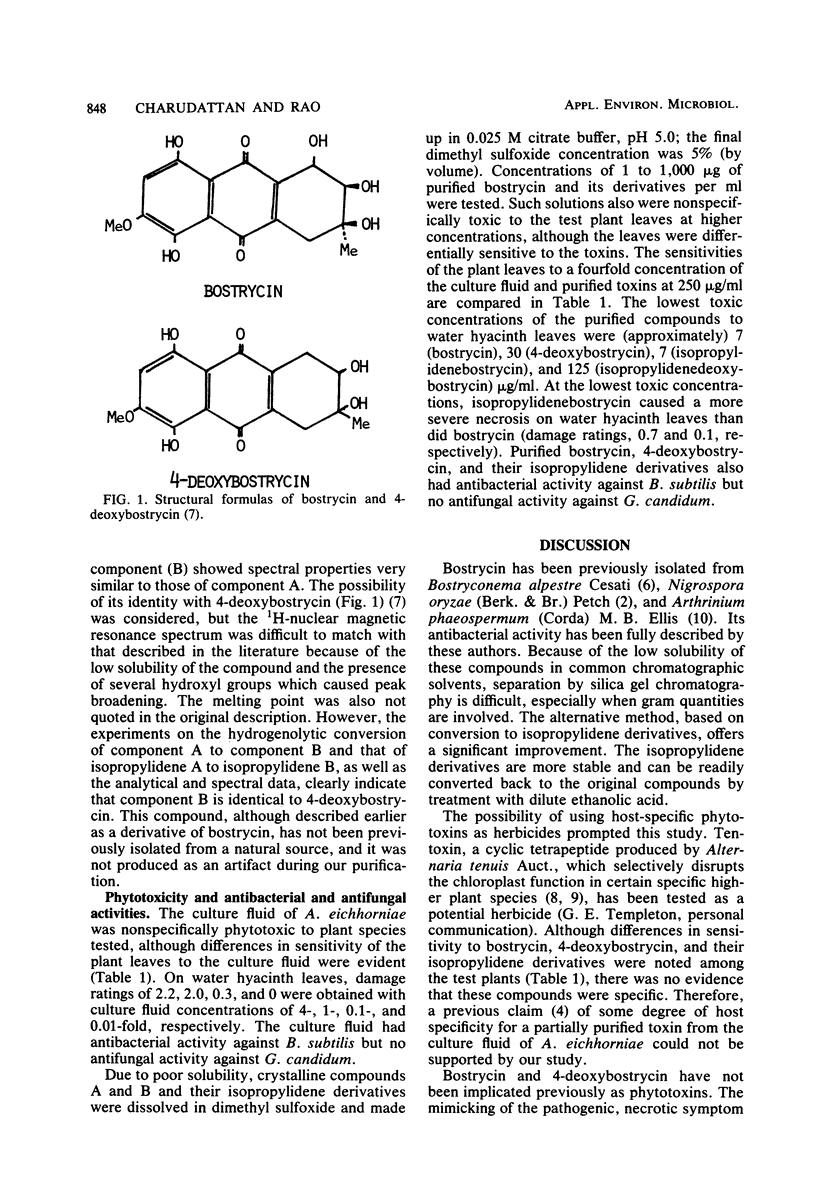
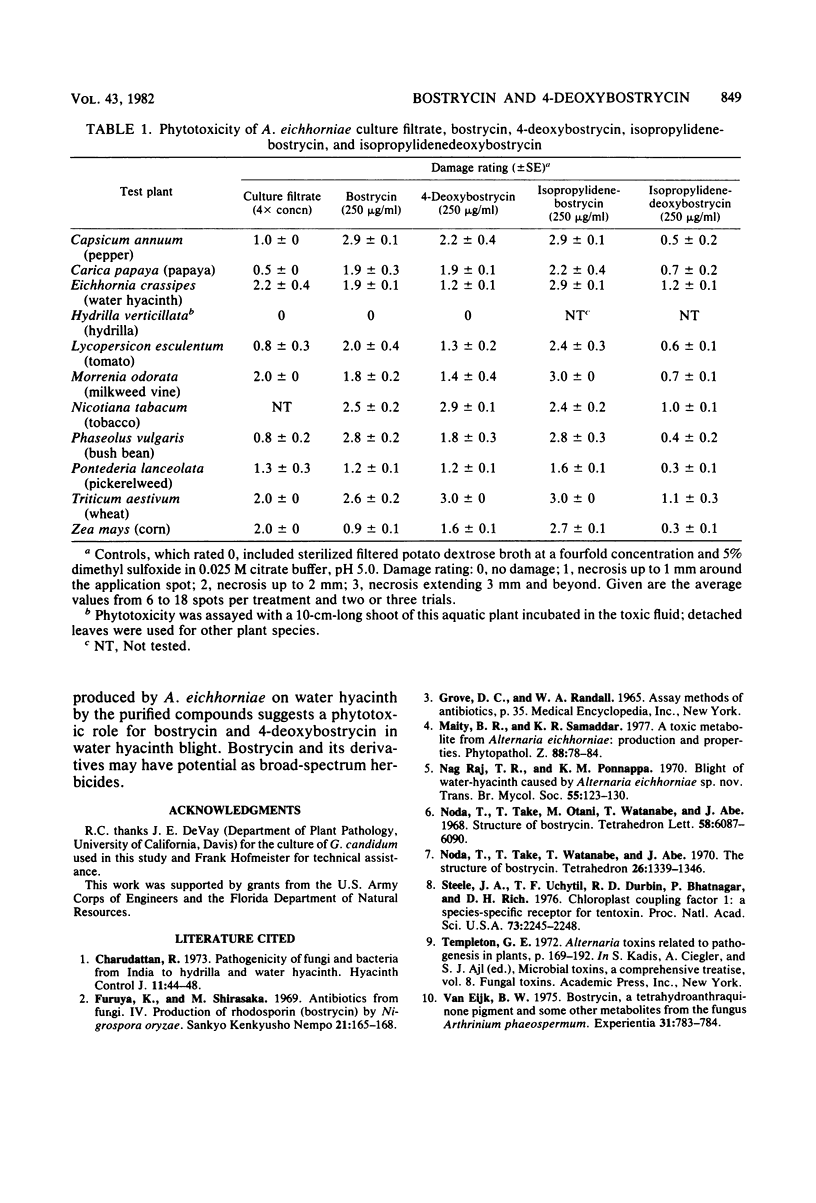
Two crystalline red pigments with phytotoxic activity were isolated from culture filtrates of Alternaria eichhorniae, a pathogen of the water hyacinth Eichhornia crassipes. The pigments were present in the ratio of 4:1 and were identified as bostrycin and 4-deoxybostrycin, respectively. This is the first isolation of 4-deoxybostrycin from a natural source. Bostrycin, 4-deoxybostrycin, and their isopropylidene derivatives induced necrosis on tested plant leaves comparable to the A. eichhorniae-induced necrosis on water hyacinth. The lowest phytotoxic concentrations of crystalline bostrycin and 4-deoxybostrycin on water hyacinth leaves were about 7 and 30 microgram/ml, respectively. Both substances were inhibitory to Bacillus subtilis but were inactive against the fungus Geotrichum candidum.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Noda T., Take T., Otani M., Miyauchi K., Watanabe T., Abe J. Structure of bostrycin. Tetrahedron Lett. 1968 Dec;(58):6087–6090. doi: 10.1016/s0040-4039(00)70801-x. [DOI] [PubMed] [Google Scholar]
- Noda T., Take T., Watanabe T., Abe J. The structure of bostrycin. Tetrahedron. 1970 Mar;26(5):1339–1346. doi: 10.1016/s0040-4020(01)93004-2. [DOI] [PubMed] [Google Scholar]
- Steele J. A., Uchytil T. F., Durbin R. D., Bhatnagar P., Rich D. H. Chloroplast coupling factor 1: A species-specific receptor for tentoxin. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2245–2248. doi: 10.1073/pnas.73.7.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]


