Abstract
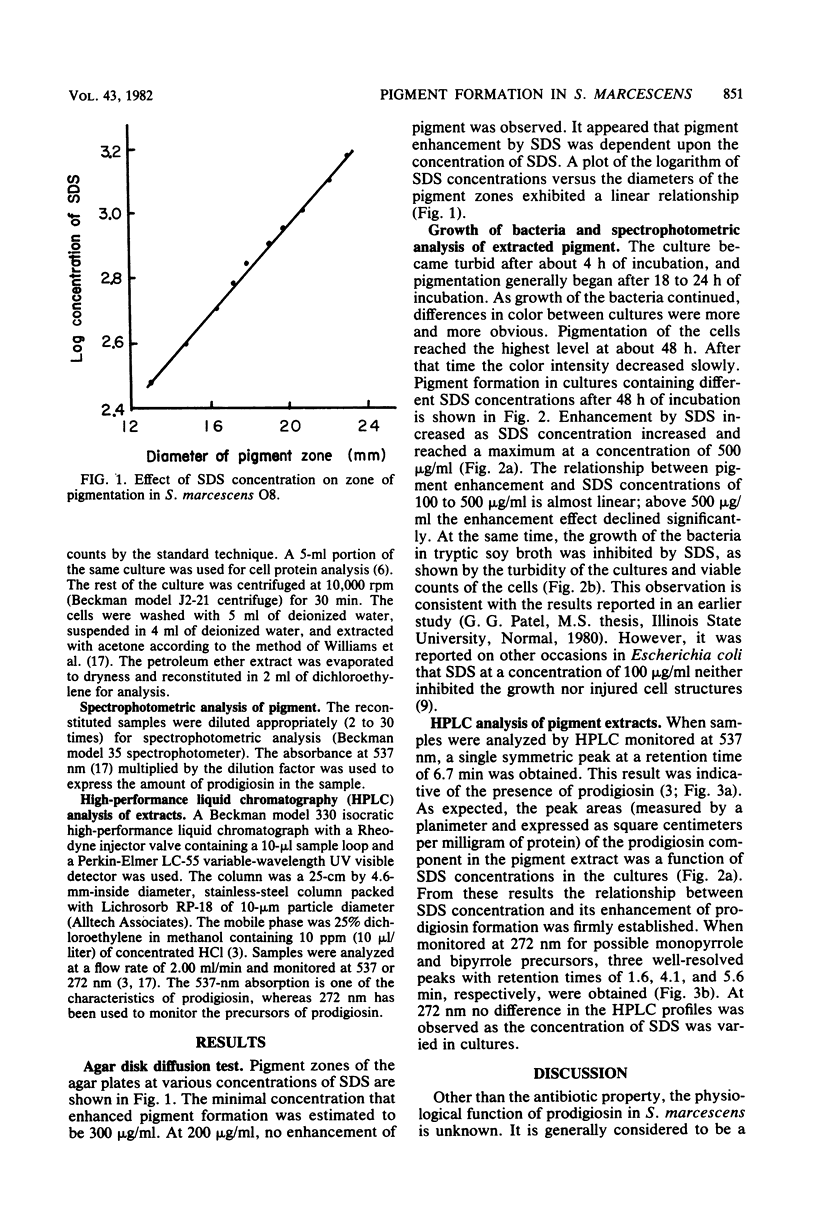
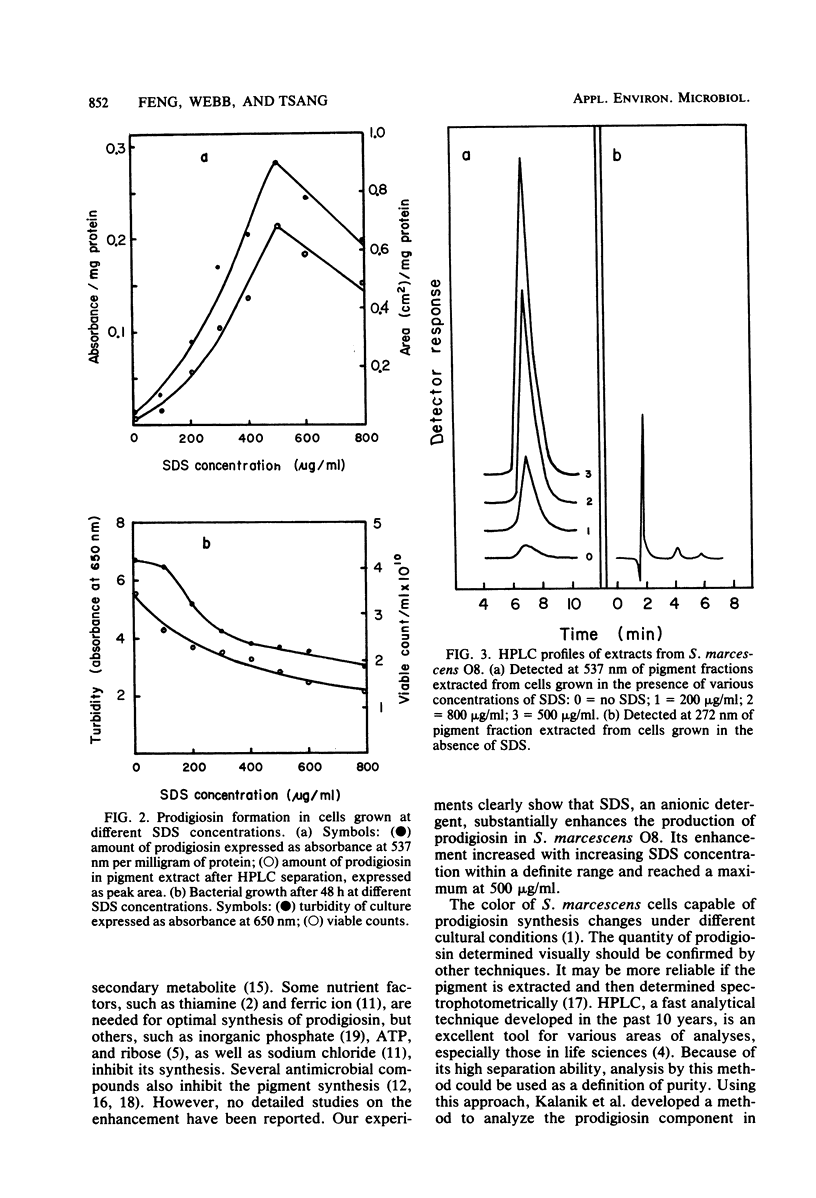
Three methods were used to determine the enhancement by sodium dodecyl sulfate (SDS) of prodigiosin formation in Serratia marcescens O8. The results of the agar disk diffusion method indicated that pigment formation was dependent upon the concentration of SDS. Diameters of the pigment zones were proportional to the logarithm of SDS concentrations of 300 to 1,500 μg/ml. When bacteria were grown in broth containing SDS from 0 to 800 μg/ml and the pigment extracts were analyzed spectrophotometrically, a similar enhancement of pigment formation was observed. Finally, these results were confirmed by high-performance liquid chromatographic analysis of the extracts. Prodigiosin appeared to be the sole component with increased synthesis. The possible mechanism of the SDS enhancement effect could be explained by an increase in negative binding sites by the association of SDS with a cell envelope component(s). These binding sites may be required for prodigiosin synthesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen E. G. Conditions of the colour change of prodigiosin. Nature. 1967 Dec 2;216(5118):929–931. doi: 10.1038/216929a0. [DOI] [PubMed] [Google Scholar]
- Goldschmidt M. C., Williams R. P. Thiamine-induced formation of the monopyrrole moiety of prodigiosin. J Bacteriol. 1968 Sep;96(3):609–616. doi: 10.1128/jb.96.3.609-616.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawanson A. O., Sholeye F. O. Inhibition of prodigiosin formation in Serratia marcescens by adenosine triphosphate. Experientia. 1976 Apr 15;32(4):439–440. doi: 10.1007/BF01920782. [DOI] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- SHAFA F., SALTON M. R. Disaggregation of bacterial cell walls by anionic detergents. J Gen Microbiol. 1960 Aug;23:137–141. doi: 10.1099/00221287-23-1-137. [DOI] [PubMed] [Google Scholar]
- Scandella C. J., Kornberg A. A membrane-bound phospholipase A1 purified from Escherichia coli. Biochemistry. 1971 Nov 23;10(24):4447–4456. doi: 10.1021/bi00800a015. [DOI] [PubMed] [Google Scholar]
- Scheusner D. L., Busta F. F., Speck M. L. Inhibition of injured Escherichia coli by several selective agents. Appl Microbiol. 1971 Jan;21(1):46–49. doi: 10.1128/am.21.1.46-49.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M. P., Munoz E. F. Effect of iron and salt on prodigiosin synthesis in Serratia marcescens. J Bacteriol. 1973 Jun;114(3):999–1006. doi: 10.1128/jb.114.3.999-1006.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang J. C., Sheung X. Inhibitory effect of polymyxin B on prodigiosin biosynthesis in Serratia marcescens. J Antibiot (Tokyo) 1980 Apr;33(4):455–457. doi: 10.7164/antibiotics.33.455. [DOI] [PubMed] [Google Scholar]
- Tsang J. C., Tattrie S., Kallvy D. Outer cell envelope glycoprotein from two strains of Serratia marcescens. Appl Microbiol. 1971 Jan;21(1):27–31. doi: 10.1128/am.21.1.27-31.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS R. P., GREEN J. A., RAPPO-PORT D. A. Studies on pigmentation of Serratia marcescens. I. Spectral and paper chromatographic properties of prodigiosin. J Bacteriol. 1956 Jan;71(1):115–120. doi: 10.1128/jb.71.1.115-120.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinbaum G., Markman R. A rapid technique for distinguishing enzymatically active proteins in the cell"envelope" of Escherichia coli B. Biochim Biophys Acta. 1966 Jul 27;124(1):207–209. doi: 10.1016/0304-4165(66)90335-7. [DOI] [PubMed] [Google Scholar]
- Williams R. P. Biosynthesis of prodigiosin, a secondary metabolite of Serratia marcescens. Appl Microbiol. 1973 Mar;25(3):396–402. doi: 10.1128/am.25.3.396-402.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. P., Gott C. L. Inhibition by streptomycin of the biosynthesis of prodigiosin. Biochem Biophys Res Commun. 1964 May 22;16(1):47–52. doi: 10.1016/0006-291x(64)90209-8. [DOI] [PubMed] [Google Scholar]
- Witney F. R., Failla M. L., Weinberg E. D. Phosphate inhibition of secondary metabolism in Serratia marcescens. Appl Environ Microbiol. 1977 May;33(5):1042–1046. doi: 10.1128/aem.33.5.1042-1046.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu V. L. Serratia marcescens: historical perspective and clinical review. N Engl J Med. 1979 Apr 19;300(16):887–893. doi: 10.1056/NEJM197904193001604. [DOI] [PubMed] [Google Scholar]


