Abstract
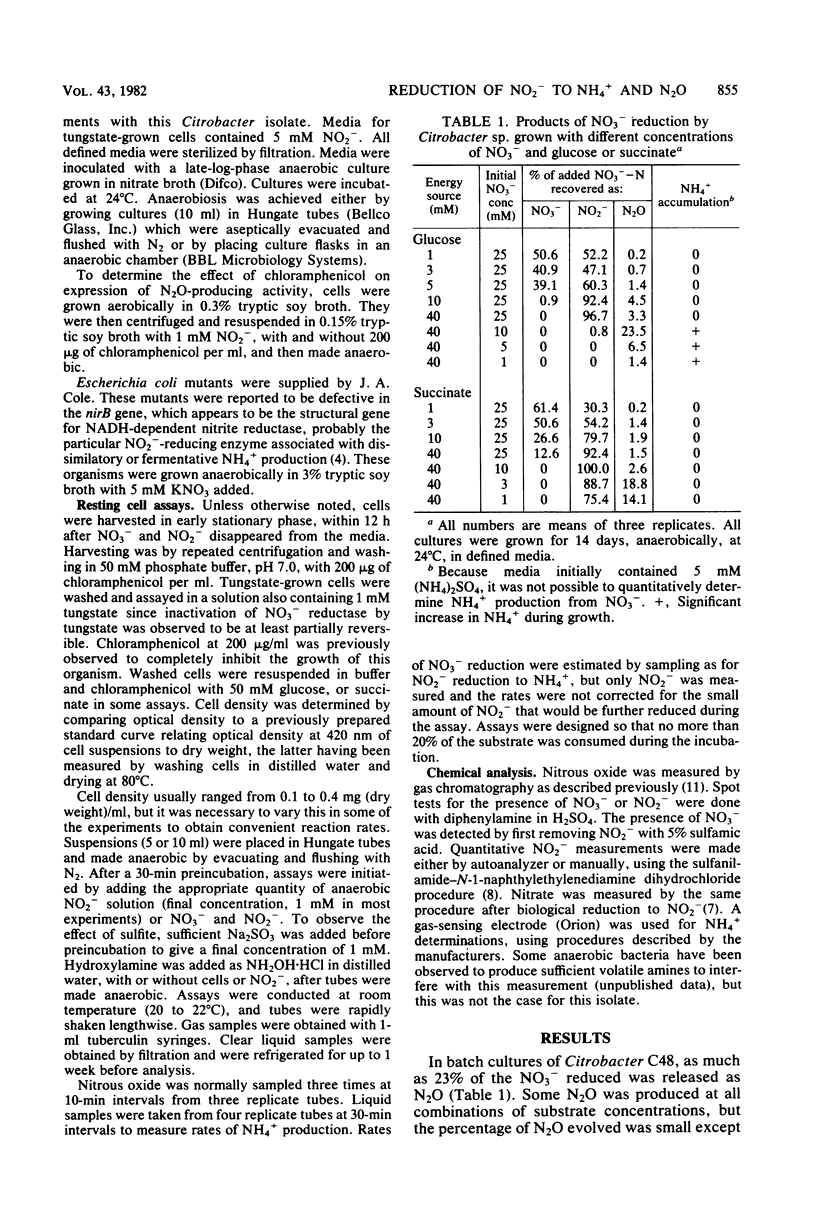
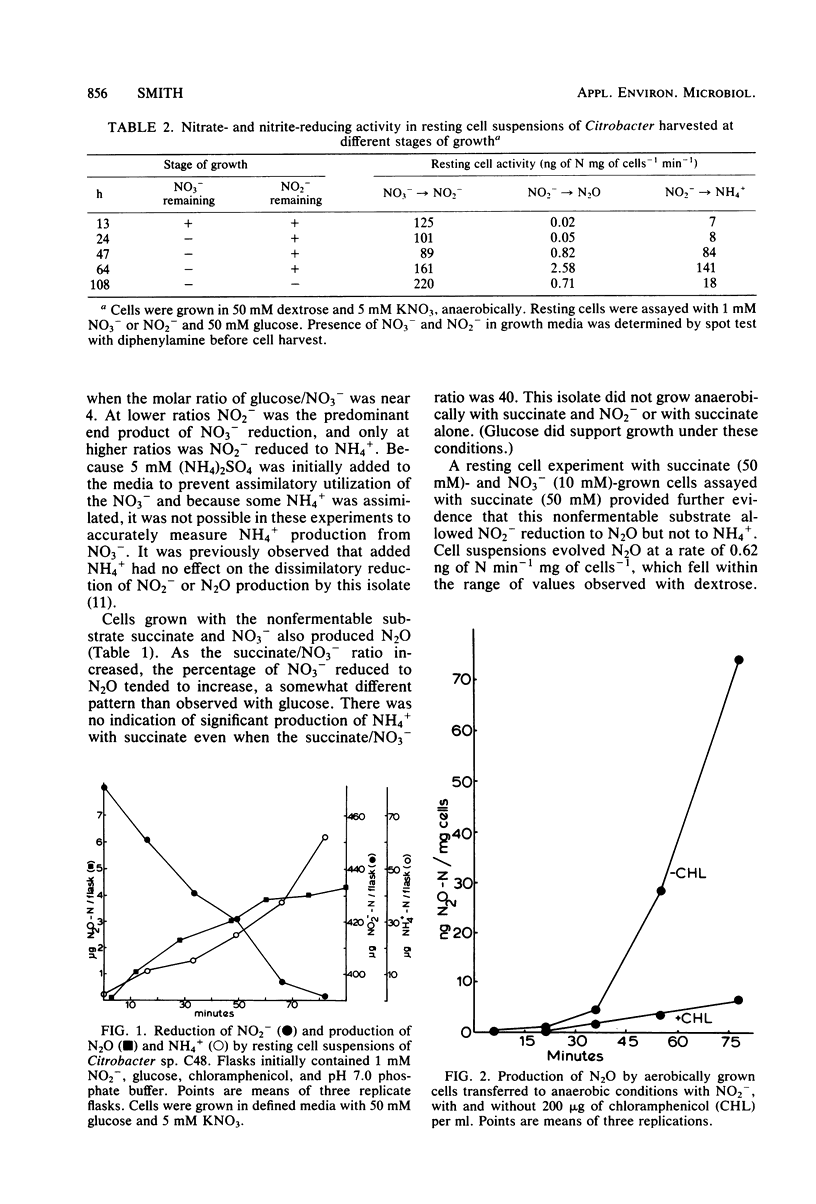
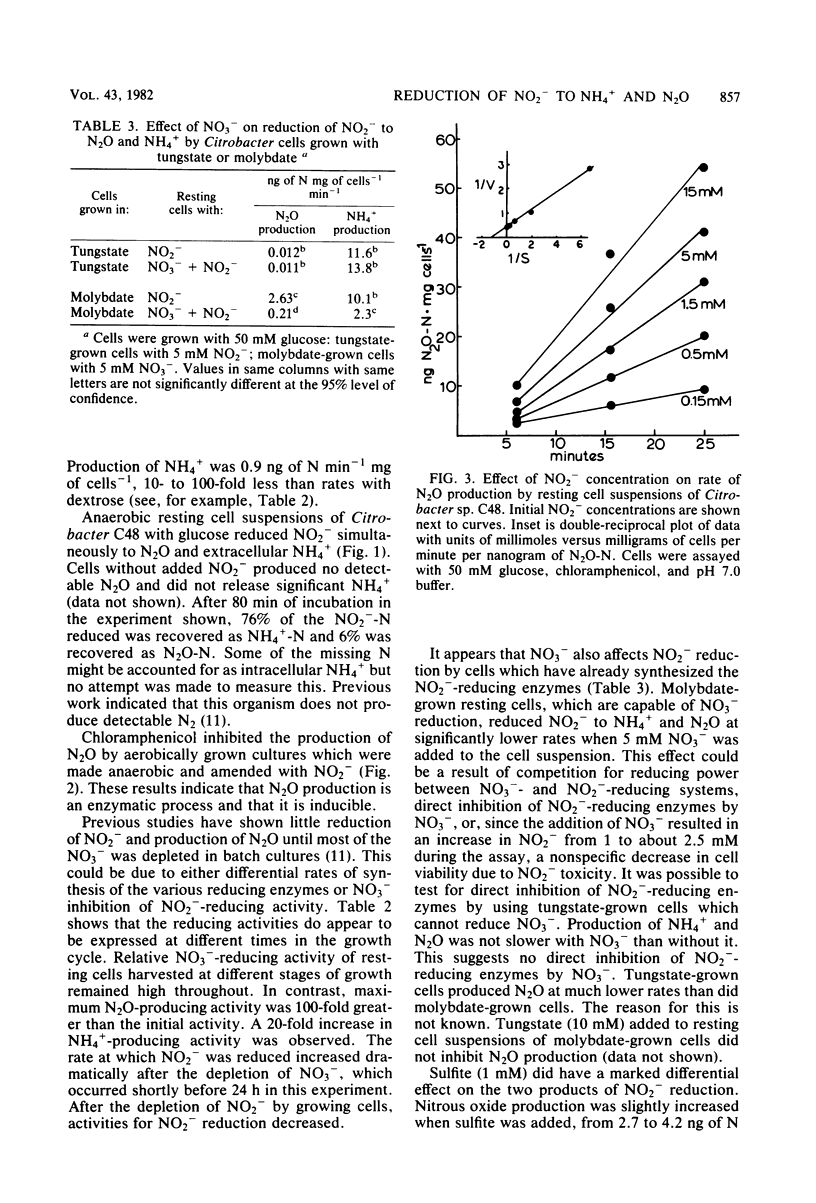
Dissimilatory reduction of NO2− to N2O and NH4+ by a soil Citrobacter sp. was studied in an attempt to elucidate the physiological and ecological significance of N2O production by this mechanism. In batch cultures with defined media, NO2− reduction to NH4+ was favored by high glucose and low NO3− concentrations. Nitrous oxide production was greatest at high glucose and intermediate NO3− concentrations. With succinate as the energy source, little or no NO2− was reduced to NH4+ but N2O was produced. Resting cell suspensions reduced NO2− simultaneously to N2O and free extracellular NH4+. Chloramphenicol prevented the induction of N2O-producing activity. The Km for NO2− reduction to N2O was estimated to be 0.9 mM NO2−, yet the apparent Km for overall NO2− reduction was considerably lower, no greater than 0.04 mM NO2−. Activities for N2O and NH4+ production increased markedly after depletion of NO3− from the media. Amendment with NO3− inhibited N2O and NH4+ production by molybdate-grown cells but not by tungstate-grown cells. Sulfite inhibited production of NH4+ but not of N2O. In a related experiment, three Escherichia coli mutants lacking NADH-dependent nitrite reductase produced N2O at rates equal to the wild type. These observations suggest that N2O is produced enzymatically but not by the same enzyme system responsible for dissimilatory reduction of NO2− to NH4+.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Lowe R. H., Gillespie M. C. An Escherichia coli strain for use in nitrate analysis. J Agric Food Chem. 1975 Jul-Aug;23(4):783–785. doi: 10.1021/jf60200a018. [DOI] [PubMed] [Google Scholar]
- Payne W. J. Reduction of nitrogenous oxides by microorganisms. Bacteriol Rev. 1973 Dec;37(4):409–452. doi: 10.1128/br.37.4.409-452.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R. H., DeMoss J. A. Formation of the formate-nitrate electron transport pathway from inactive components in Escherichia coli. J Bacteriol. 1976 Apr;126(1):478–486. doi: 10.1128/jb.126.1.478-486.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen J. Capacity for denitrification and reduction of nitrate to ammonia in a coastal marine sediment. Appl Environ Microbiol. 1978 Feb;35(2):301–305. doi: 10.1128/aem.35.2.301-305.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


