Abstract
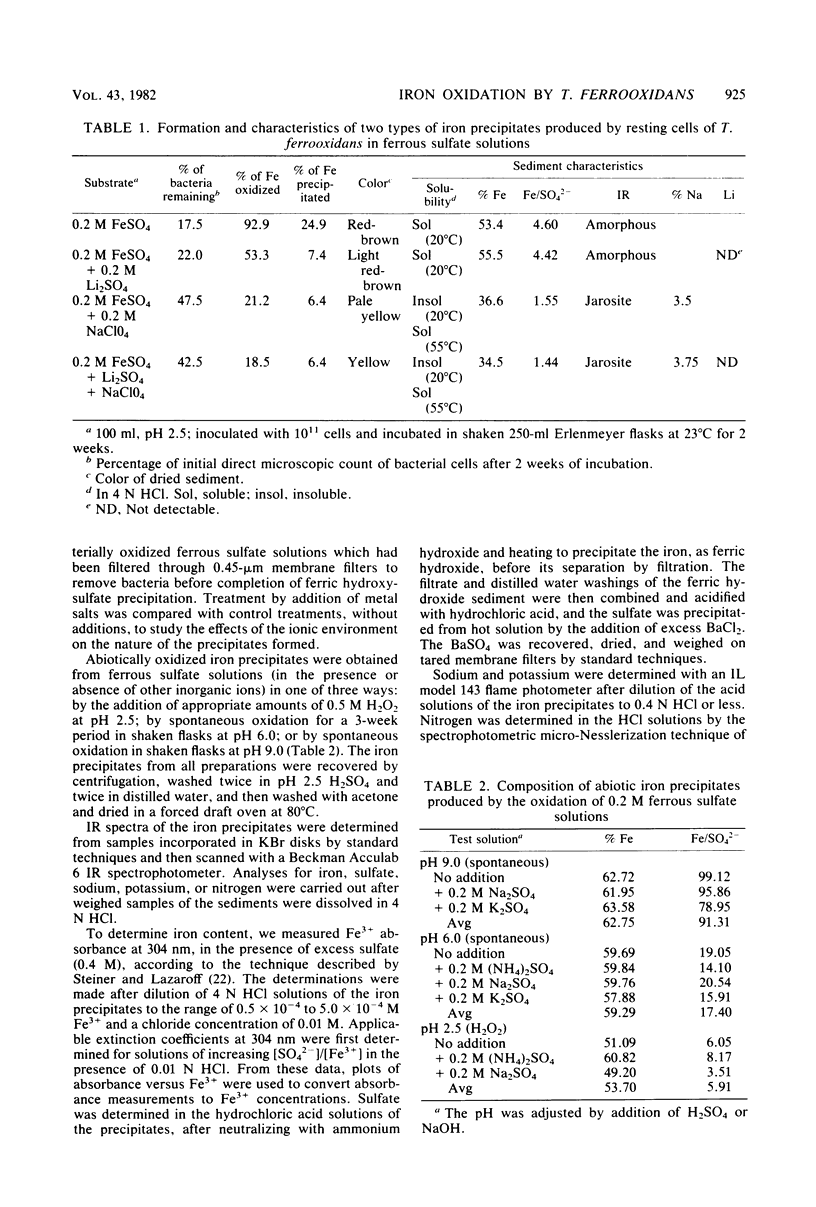
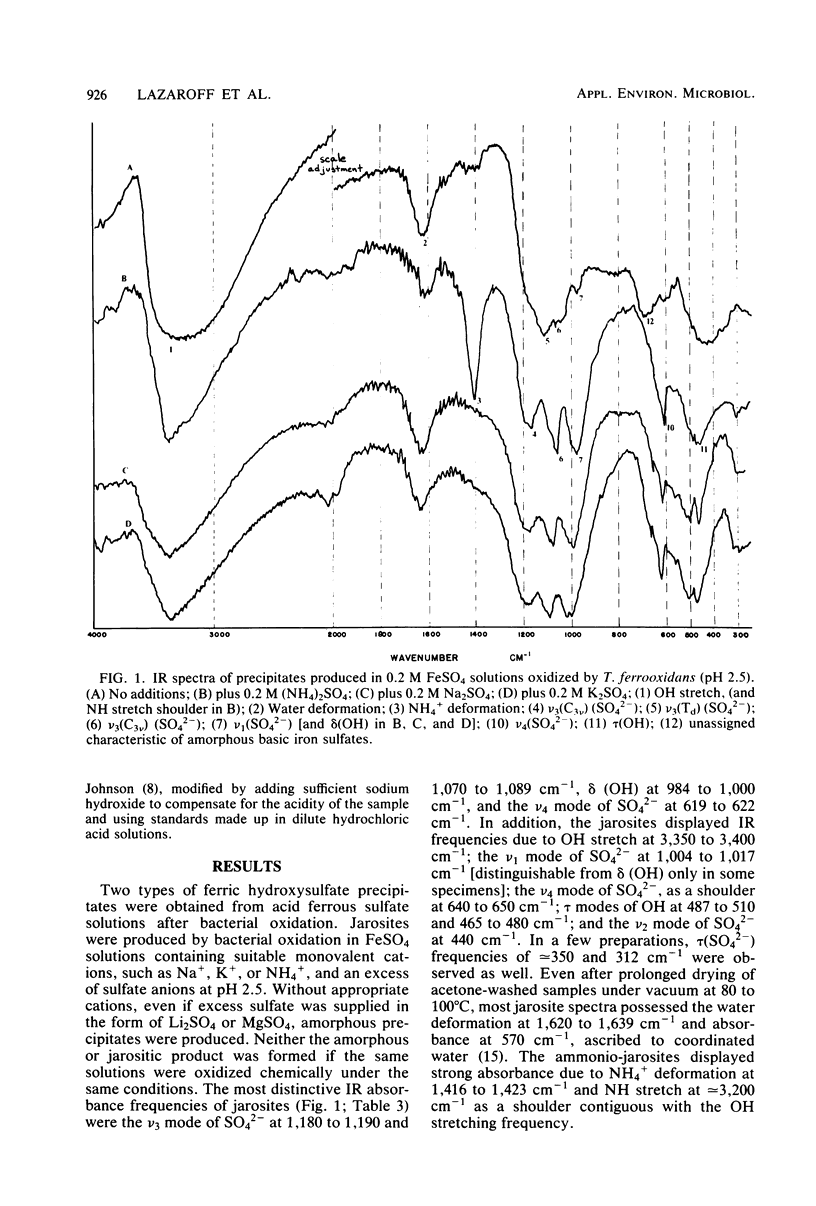
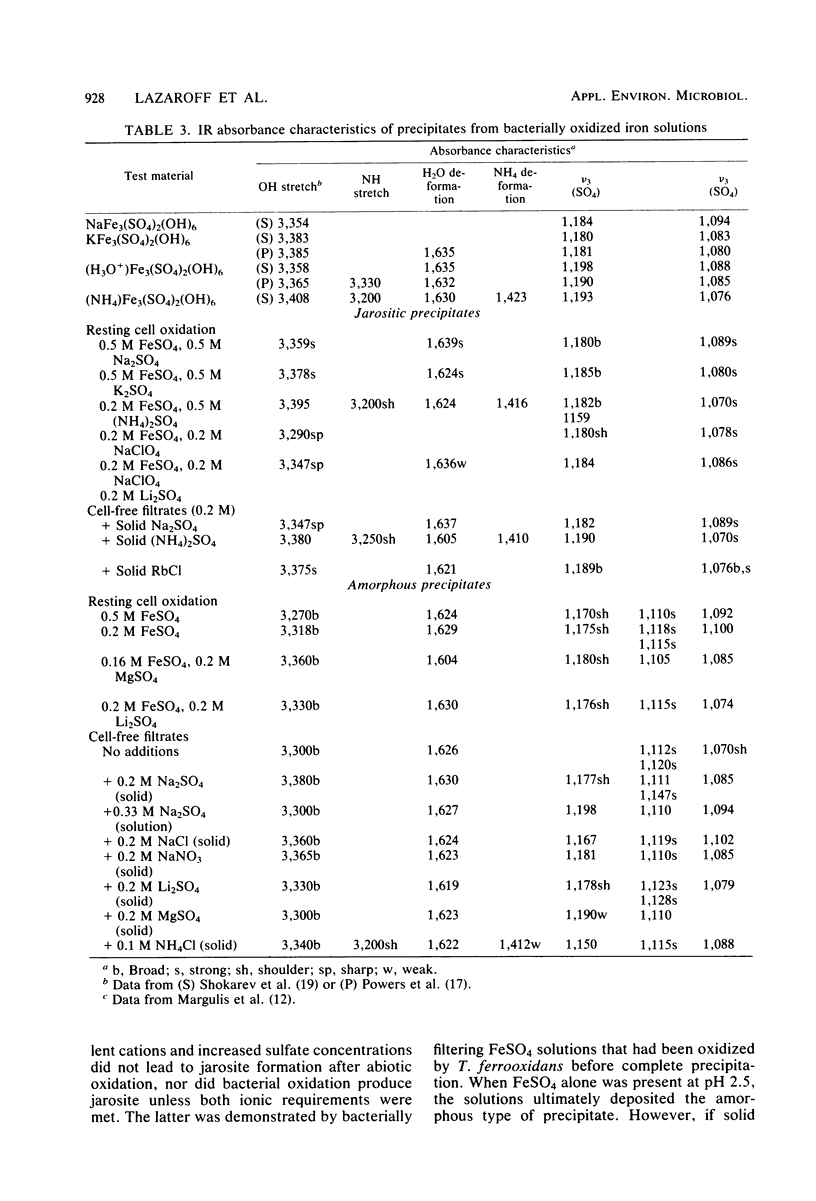
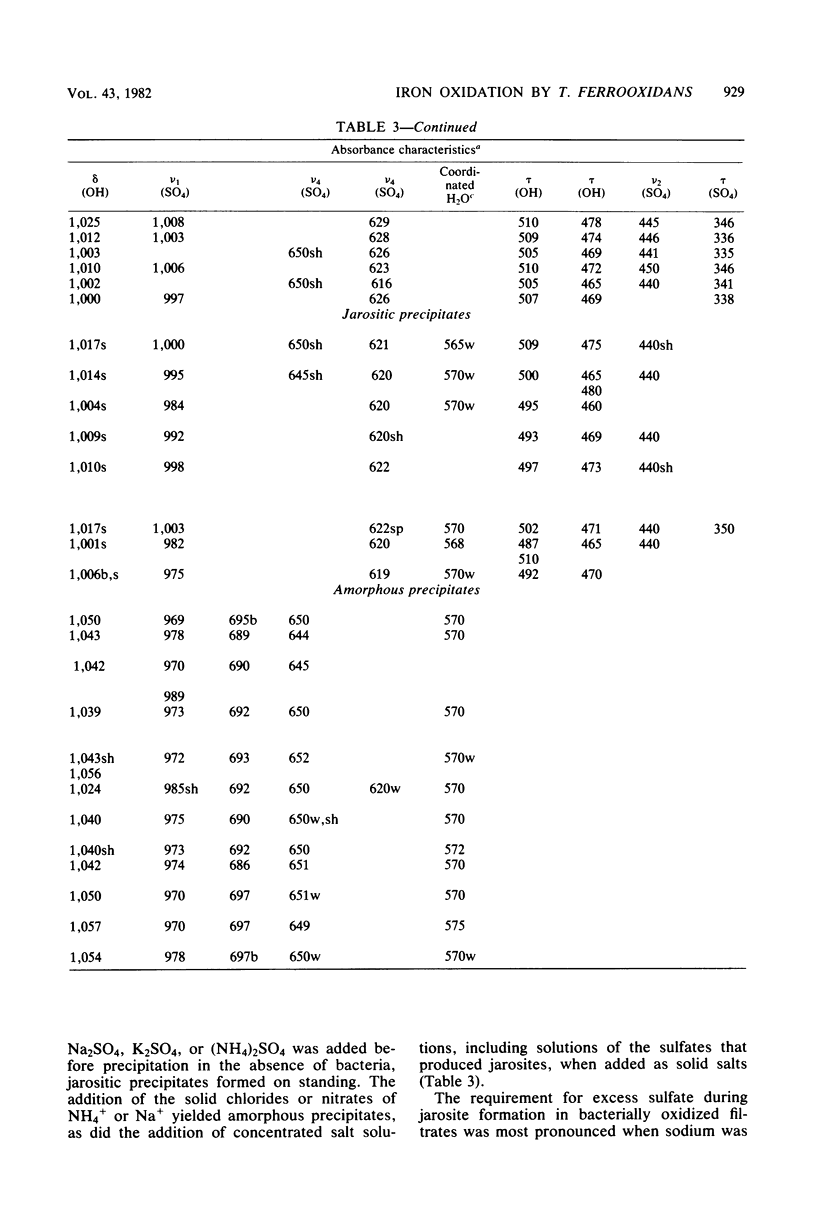
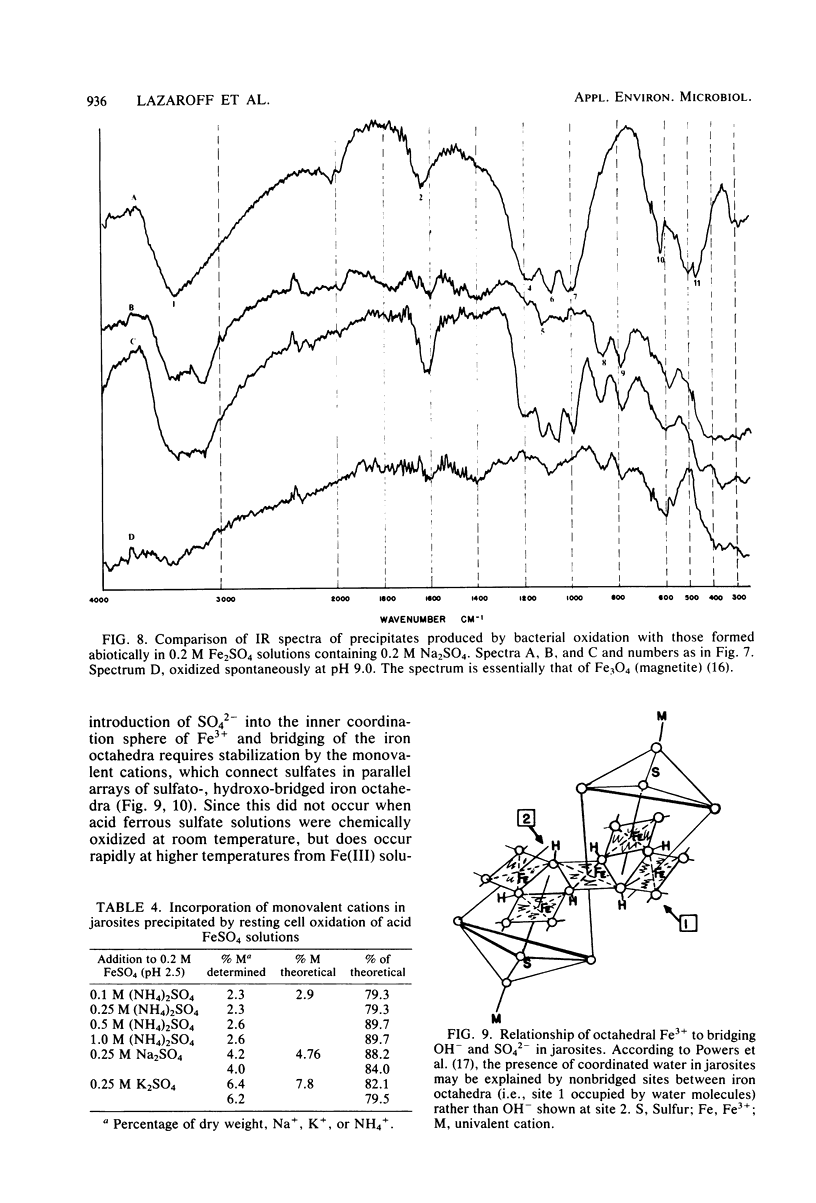
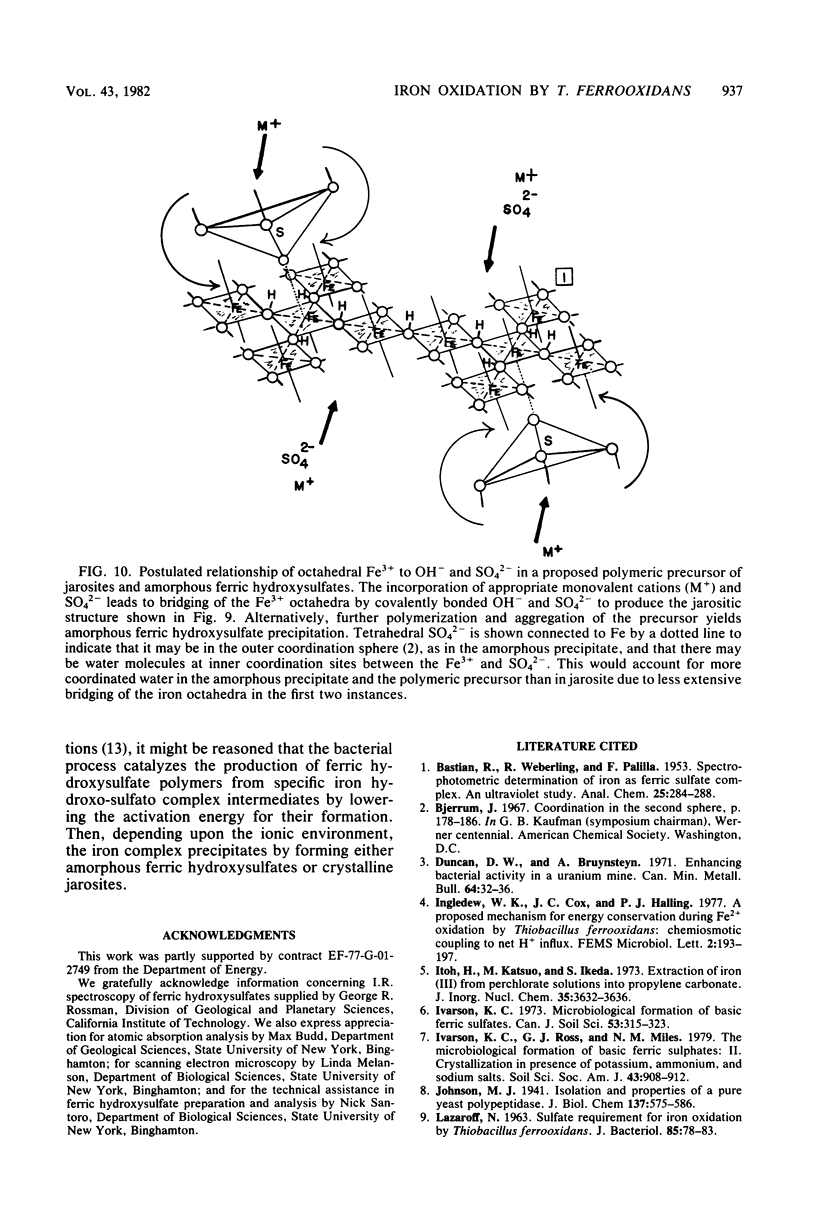
The oxidation of ferrous ions, in acid solution, by resting suspensions of Thiobacillus ferrooxidans produced sediments consisting of crystalline jarosites, amorphous ferric hydroxysulfates, or both. These products differed conspicuously in chemical composition and infrared spectra from precipitates formed by abiotic oxidation under similar conditions. The amorphous sediments, produced by bacterial oxidation, exhibited a distinctive fibroporous microstructure when examined by scanning electron microscopy. Infrared spectra indicated outer-sphere coordination of Fe(III) by sulfate ions, as well as inner-sphere coordination by water molecules and bridging hydroxo groups. In the presence of excess sulfate and appropriate monovalent cations, jarosites, instead of amorphous ferric hydroxysulfates, precipitated from bacterially oxidized iron solutions. It is proposed that the jarositic precipitates result from the conversion of outer-sphere (Td) sulfate, present in a soluble polymeric Fe(III) complex, to inner-sphere (C3v) bridging sulfate. The amorphous precipitates result from the further polymerization of hydroxo-linked iron octahedra and charge stabilized aggregation of the resulting iron complexes in solution. This view was supported by observations that bacterially oxidized iron solutions gave rise to either amorphous or jarositic sediments in response to ionic environments imposed after oxidation had been completed and the bacteria had been removed by filtration.
Full text
PDF



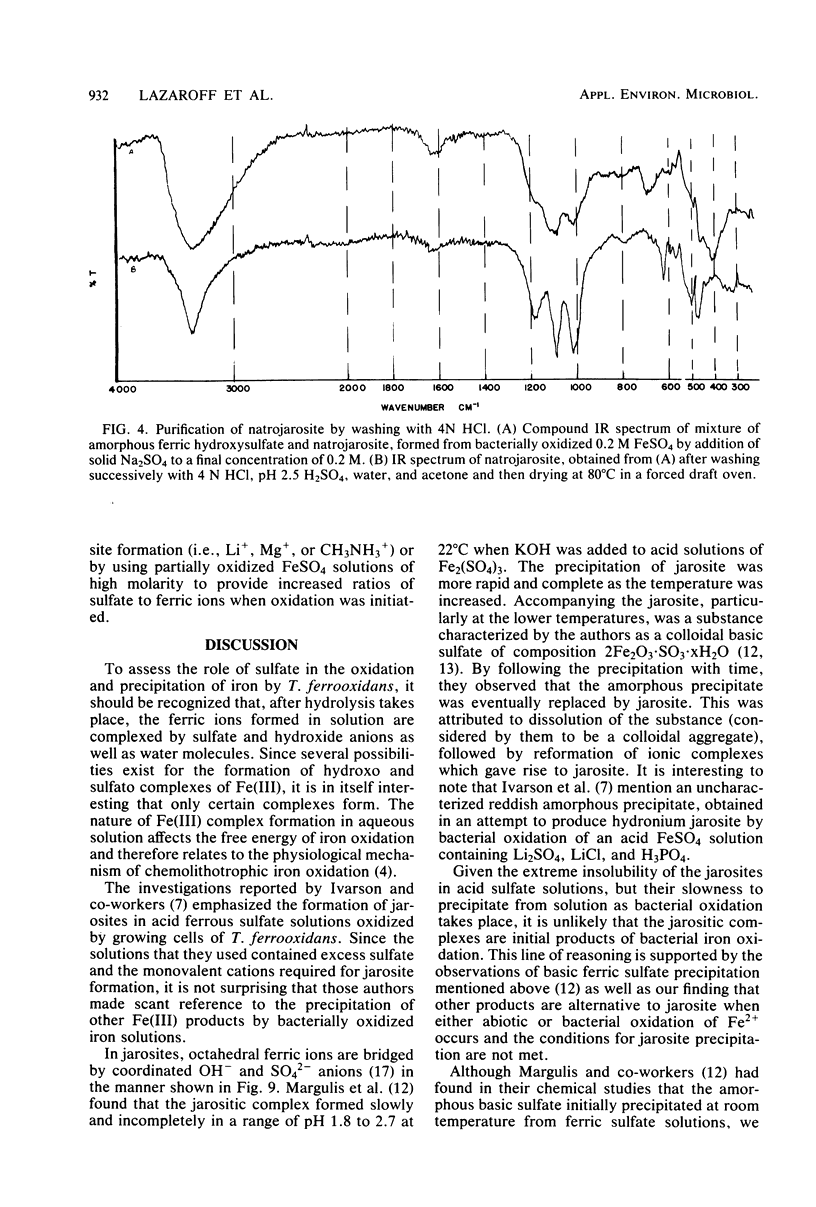
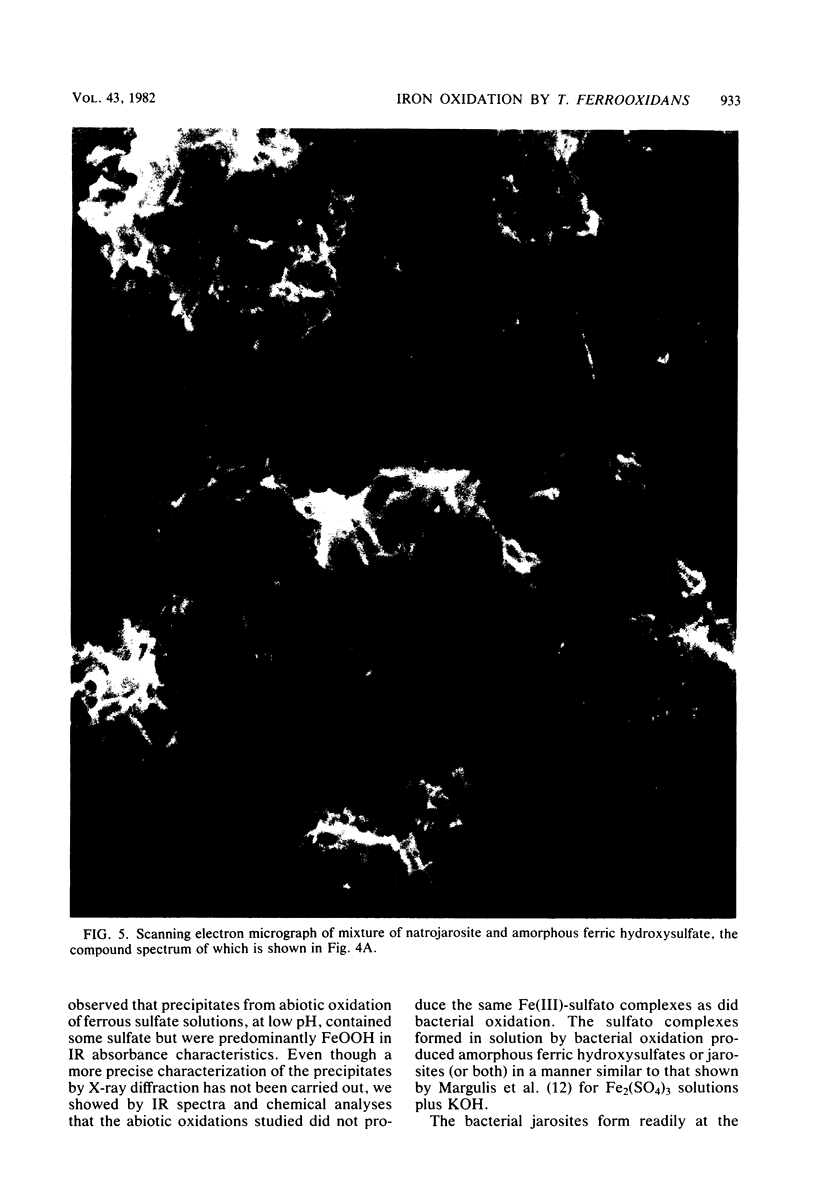
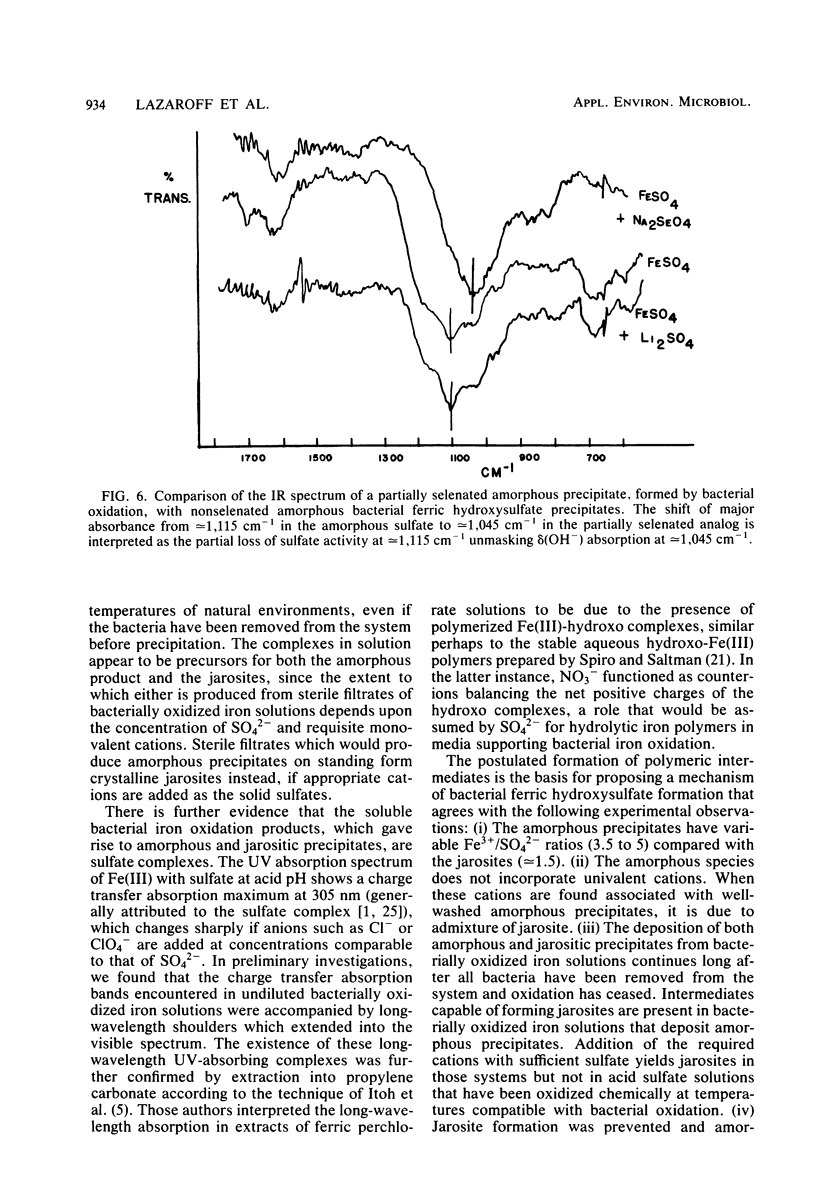
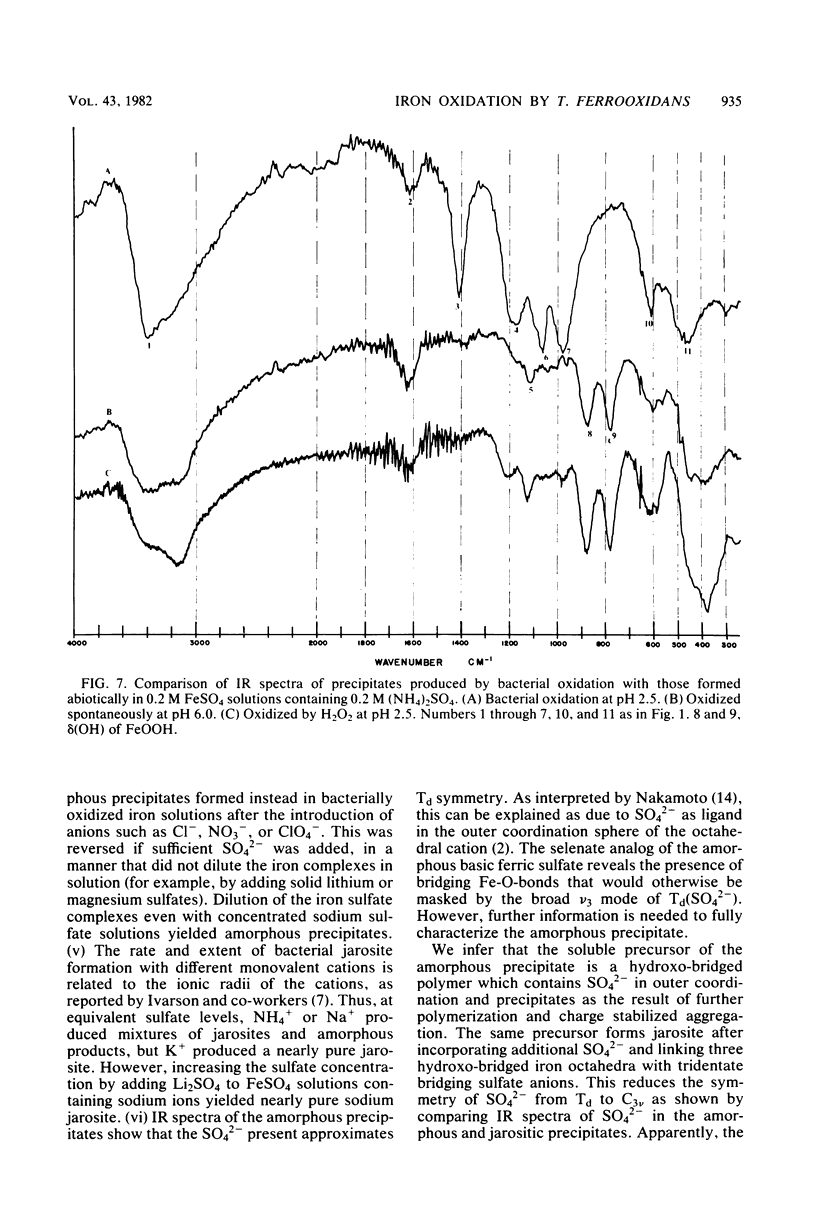

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- LEATHEN W. W., BRALEY S. A., Sr, MCINTYRE L. D. The role of bacteria in the formation of acid from certain sulfuritic constituents associated with bituminous coal. II. Ferrous iron oxidizing bacteria. Appl Microbiol. 1953 Mar;1(2):65–68. doi: 10.1128/am.1.2.65-68.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaroff N. SULFATE REQUIREMENT FOR IRON OXIDATION BY THIOBACILLUS FERROOXIDANS. J Bacteriol. 1963 Jan;85(1):78–83. doi: 10.1128/jb.85.1.78-83.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILVERMAN M. P., LUNDGREN D. G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. I. An improved medium and a harvesting procedure for securing high cell yields. J Bacteriol. 1959 May;77(5):642–647. doi: 10.1128/jb.77.5.642-647.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A., Korczynski M. S., Lundgren D. G. Kinetic studies of iron oxidation by whole cells of Ferrobacillus ferrooxidans. J Bacteriol. 1969 Aug;99(2):552–557. doi: 10.1128/jb.99.2.552-557.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner M., Lazaroff N. Direct method for continuous determination of iron oxidation by autotrophic bacteria. Appl Microbiol. 1974 Nov;28(5):872–880. doi: 10.1128/am.28.5.872-880.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]





