Abstract
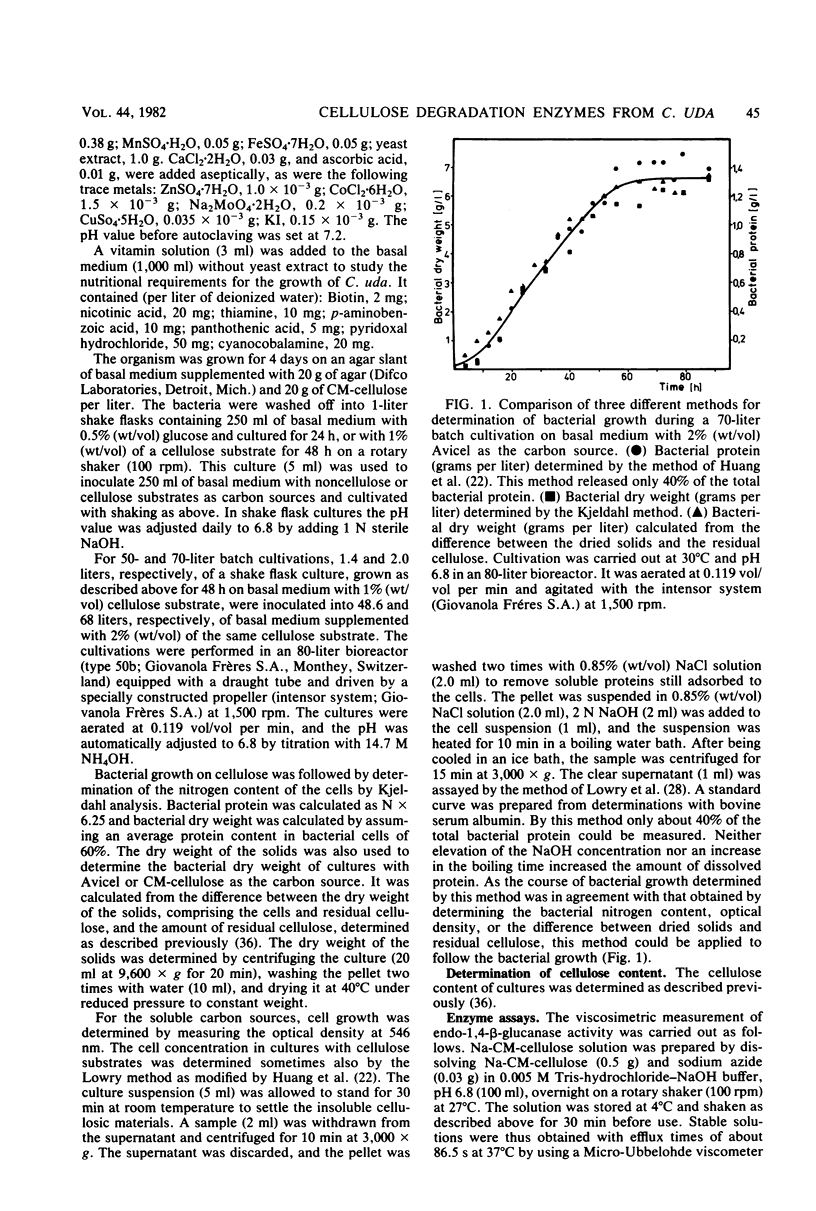
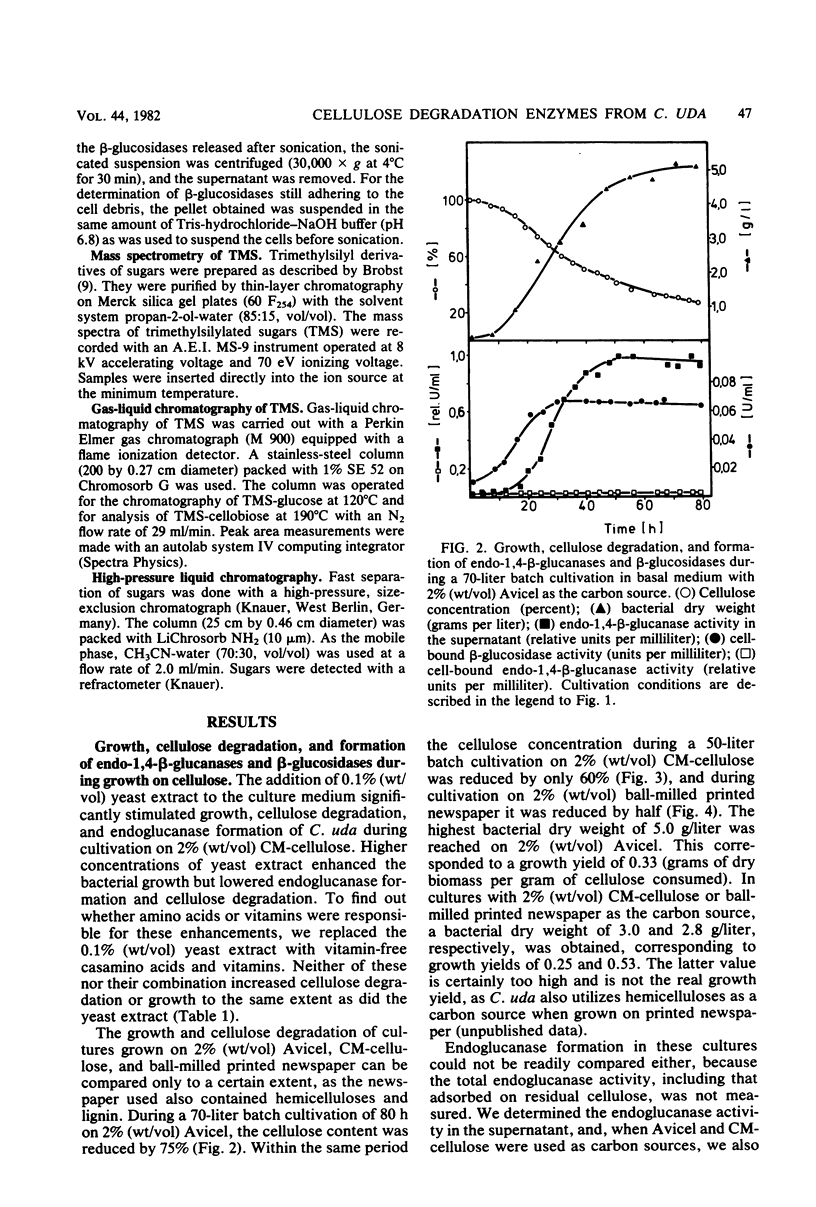
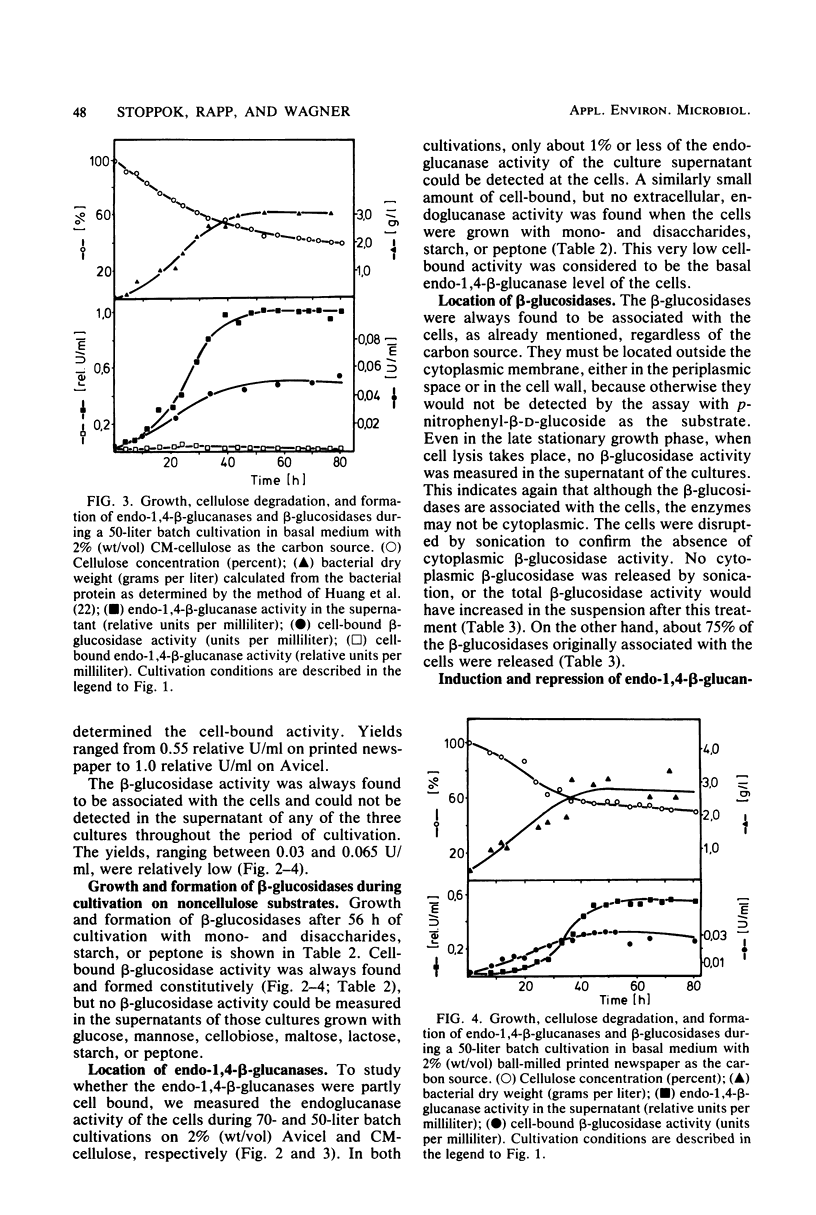
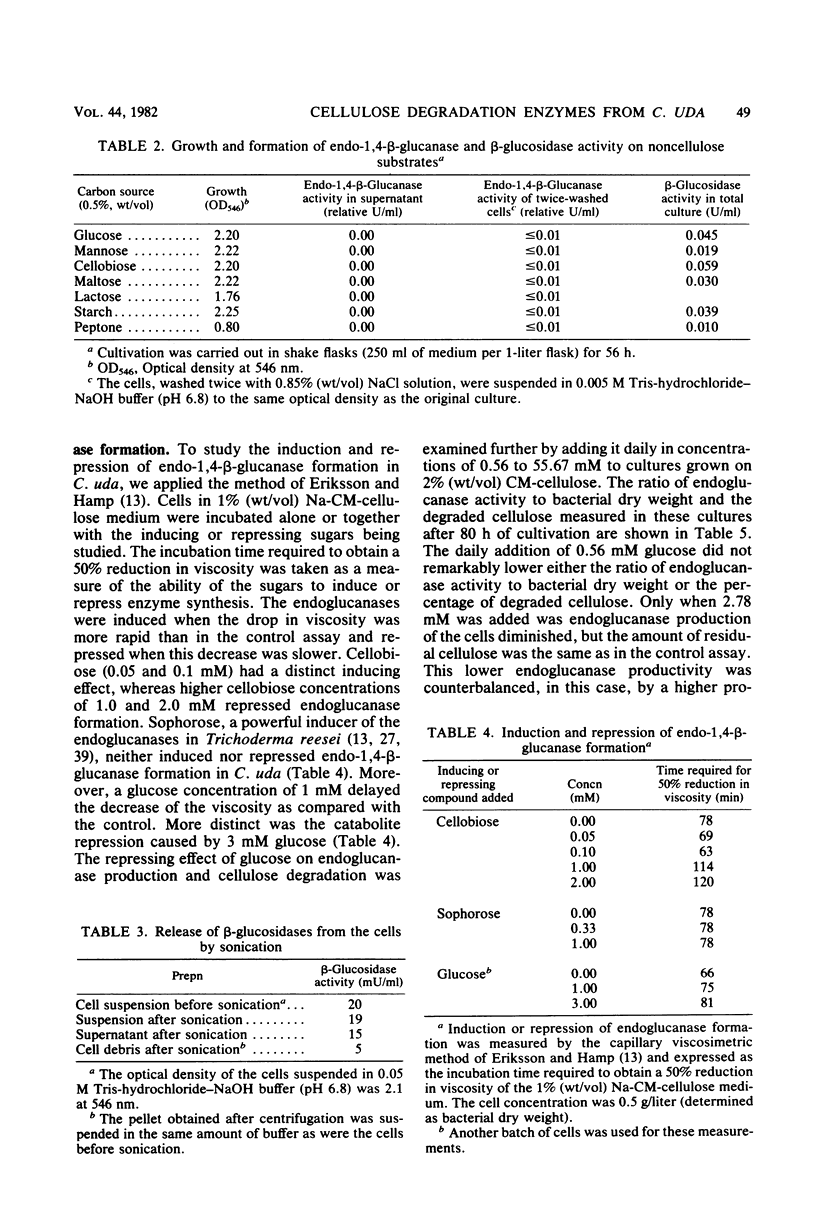
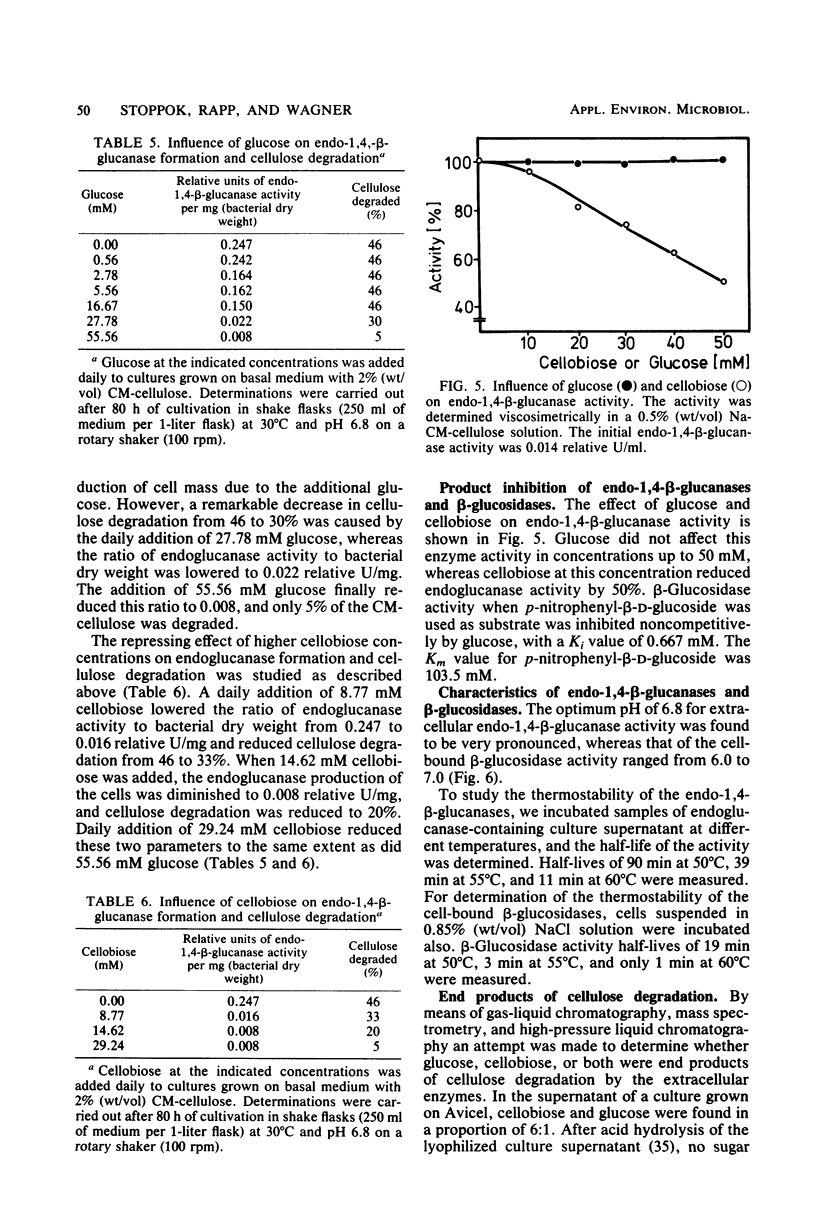
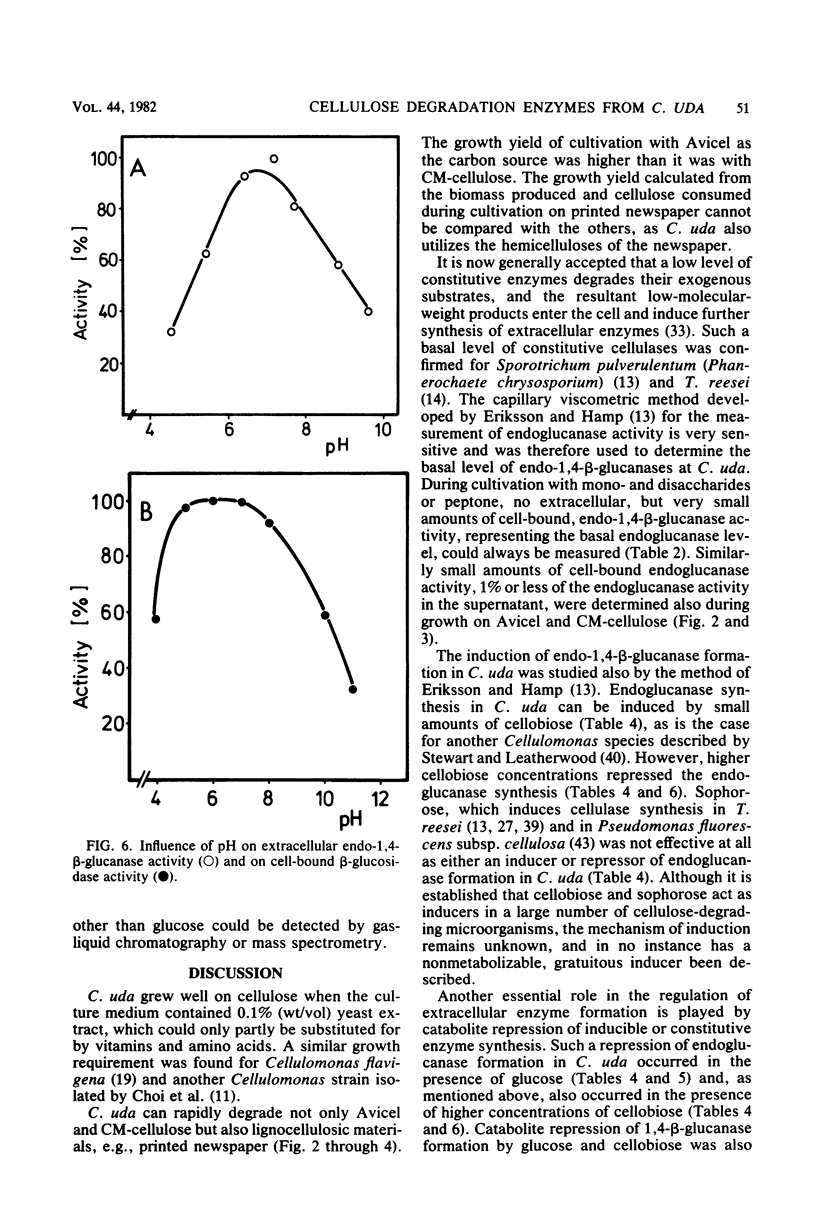
The formation and location of endo-1,4-β-glucanases and β-glucosidases were studied in cultures of Cellulomonas uda grown on microcrystalline cellulose, carboxymethyl cellulose, printed newspaper, and some mono- or disaccharides. Endo-1,4-Glucanases were found to be extracellular, but a very small amount of cell-bound endo-1,4-β-glucanase was considered to be the basal endoglucanase level of the cells. The formation of extracellular endo-1,4-β-glucanases was induced by cellobiose and repressed by glucose. Extracellular endoglucanase activity was inhibited by cellobiose but not by glucose. β-Glucosidases, on the other hand, were formed constitutively and found to be cell bound. β-Glucosidase activity was inhibited noncompetitively by glucose. Some characteristics such as the optimal pH for and the thermostability of the endoglucanases and β-glucosidases and the end products of cellulose degradation were determined.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER J. K. Characteristics of cellobiose phosphorylase. J Bacteriol. 1961 Jun;81:903–910. doi: 10.1128/jb.81.6.903-910.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J. K. Purification and specificity of cellobiose phosphorylase from Clostridium thermocellum. J Biol Chem. 1968 Jun 10;243(11):2899–2904. [PubMed] [Google Scholar]
- Aït N., Creuzet N., Cattanéo J. Characterization and purification of thermostable beta-glucosidase from Clostridium thermocellum. Biochem Biophys Res Commun. 1979 Sep 27;90(2):537–546. doi: 10.1016/0006-291x(79)91269-5. [DOI] [PubMed] [Google Scholar]
- Berg B. Cellulase location in Cellvibrio fulvus. Can J Microbiol. 1975 Jan;21(1):51–57. doi: 10.1139/m75-007. [DOI] [PubMed] [Google Scholar]
- Berghem L. E., Pettersson L. G. The mechanism of enzymatic cellulose degradation. Purification of a cellulolytic enzyme from Trichoderma viride active on highly ordered cellulose. Eur J Biochem. 1973 Aug 1;37(1):21–30. doi: 10.1111/j.1432-1033.1973.tb02952.x. [DOI] [PubMed] [Google Scholar]
- Breuil C., Kushner D. J. Cellulase induction and the use of cellulose as a preferred growth substrate by Cellvibrio gilvus. Can J Microbiol. 1976 Dec;22(12):1776–1781. doi: 10.1139/m76-264. [DOI] [PubMed] [Google Scholar]
- Béguin P., Eisen H. Purification and partial characterization of three extracellular cellulases from Cellulomonas sp. Eur J Biochem. 1978 Jul 3;87(3):525–531. doi: 10.1111/j.1432-1033.1978.tb12403.x. [DOI] [PubMed] [Google Scholar]
- Chang W. T., Thayer D. W. The cellulase system of a Cytophaga species. Can J Microbiol. 1977 Sep;23(9):1285–1292. doi: 10.1139/m77-192. [DOI] [PubMed] [Google Scholar]
- Eriksson K. E., Hamp S. G. Regulation of Endo-1,4-beta-glucanase production in Sporotrichum pulverulentum. Eur J Biochem. 1978 Sep 15;90(1):183–190. doi: 10.1111/j.1432-1033.1978.tb12589.x. [DOI] [PubMed] [Google Scholar]
- Han Y. W., Srinivasan V. R. Isolation and characterization of a cellulose-utilizing bacterium. Appl Microbiol. 1968 Aug;16(8):1140–1145. doi: 10.1128/am.16.8.1140-1145.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchner E. V., Leatherwood J. M. Use of a cellulase-derepressed mutant of cellulomonas in the production of a single-cell protein product from cellulose. Appl Environ Microbiol. 1980 Feb;39(2):382–386. doi: 10.1128/aem.39.2.382-386.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme M. A. Viscometric determination of carboxymethylcellulase in standard international units. Arch Biochem Biophys. 1971 Nov;147(1):49–54. doi: 10.1016/0003-9861(71)90308-0. [DOI] [PubMed] [Google Scholar]
- Hägerdal B. G., Ferchak J. D., Pye E. K. Cellulolytic Enzyme System of Thermoactinomyces sp. Grown on Microcrystalline Cellulose. Appl Environ Microbiol. 1978 Oct;36(4):606–612. doi: 10.1128/aem.36.4.606-612.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hägerdal B., Harris H., Pye E. K. Association of beta-glucosidase with intact cells of Thermoactinomyces. Biotechnol Bioeng. 1979 Mar;21(3):345–355. doi: 10.1002/bit.260210302. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee B. H., Blackburn T. H. Cellulase production by a thermophilic clostridium species. Appl Microbiol. 1975 Sep;30(3):346–353. doi: 10.1128/am.30.3.346-353.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenberg J. R., Chapman C. M. Sophorose metabolism and cellulase induction in Trichoderma. Arch Microbiol. 1977 May 13;113(1-2):61–64. doi: 10.1007/BF00428581. [DOI] [PubMed] [Google Scholar]
- Nisizawa T., Suzuki H., Nakayama M., Nisizawa K. Inductive formation of cellulase by sophorose in Trichoderma viride. J Biochem. 1971 Sep;70(3):375–385. doi: 10.1093/oxfordjournals.jbchem.a129652. [DOI] [PubMed] [Google Scholar]
- Nisizawa T., Suzuki H., Nisizawa K. Catabolite repression of cellulase formation in Trichoderma viride. J Biochem. 1972 Jun;71(6):999–1007. doi: 10.1093/oxfordjournals.jbchem.a129872. [DOI] [PubMed] [Google Scholar]
- Osmundsvag K., Goksoyr J. Cellulases from Sporocytophaga myxococcoides. Purification and Properties. Eur J Biochem. 1975 Sep 15;57(2):405–409. doi: 10.1111/j.1432-1033.1975.tb02314.x. [DOI] [PubMed] [Google Scholar]
- Priest F. G. Extracellular enzyme synthesis in the genus Bacillus. Bacteriol Rev. 1977 Sep;41(3):711–753. doi: 10.1128/br.41.3.711-753.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp P., Grote E., Wagner F. Formation and Location of 1,4-beta-Glucanases and 1,4-beta-Glucosidases from Penicillium janthinellum. Appl Environ Microbiol. 1981 Apr;41(4):857–866. doi: 10.1128/aem.41.4.857-866.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg D., Mandels G. R. Induction of cellulolytic enzymes in Trichoderma reesei by sophorose. J Bacteriol. 1979 Sep;139(3):761–769. doi: 10.1128/jb.139.3.761-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart B. J., Leatherwood J. M. Derepressed synthesis of cellulase by Cellulomonas. J Bacteriol. 1976 Nov;128(2):609–615. doi: 10.1128/jb.128.2.609-615.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzenberger F. J. Cellulase production by Thermomonospora curvata isolated from municipal solid waste compost. Appl Microbiol. 1971 Aug;22(2):147–152. doi: 10.1128/am.22.2.147-152.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane K., Suzuki H., Hirotani M., Ozawa H., Nisizawa K. Effect of nature and supply of carbon sources on cellulase formation in Pseudomonas fluorescens var. cellulosa. J Biochem. 1970 Jan;67(1):9–18. doi: 10.1093/oxfordjournals.jbchem.a129238. [DOI] [PubMed] [Google Scholar]
- Yamane K., Yoshikawa T., Suzuki H., Nisizawa K. Localization of cellulase components in Pseudomonas fluorescens var. cellulosa. J Biochem. 1971 Apr;69(4):771–780. doi: 10.1093/oxfordjournals.jbchem.a129525. [DOI] [PubMed] [Google Scholar]


