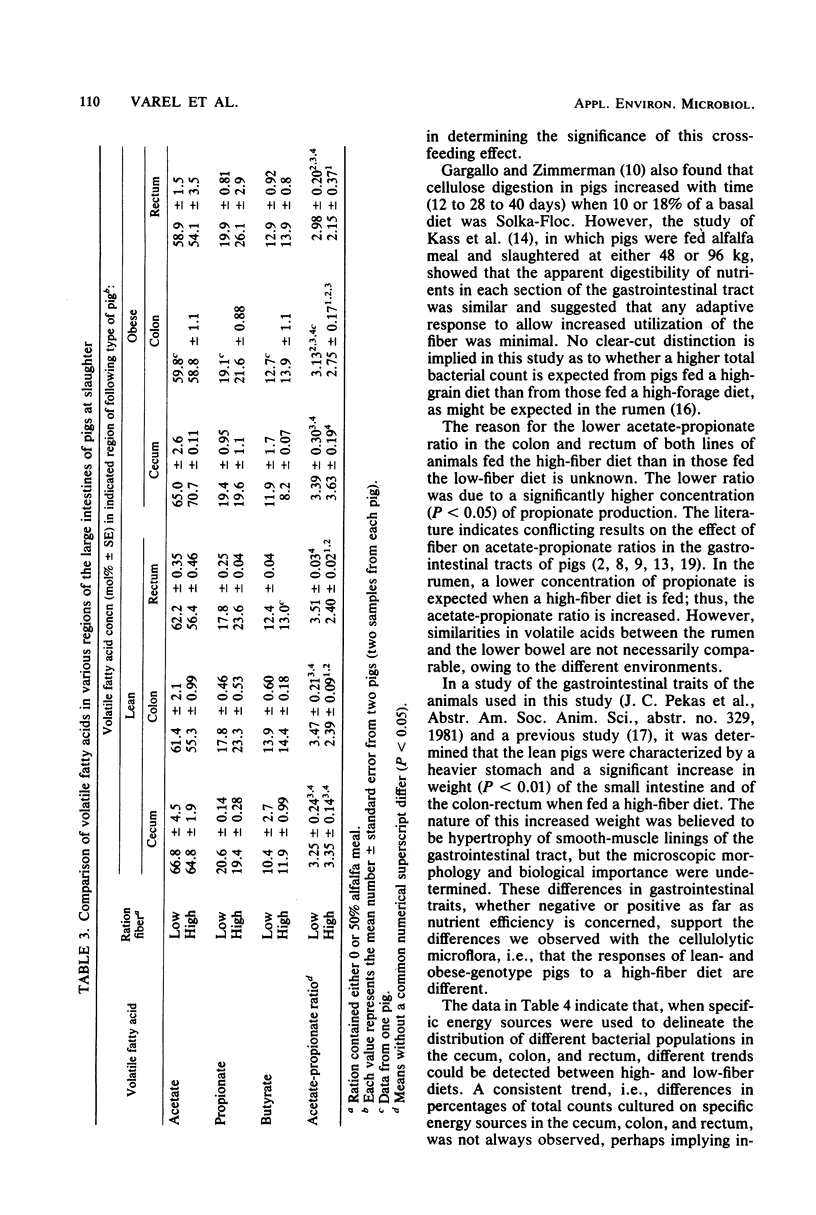
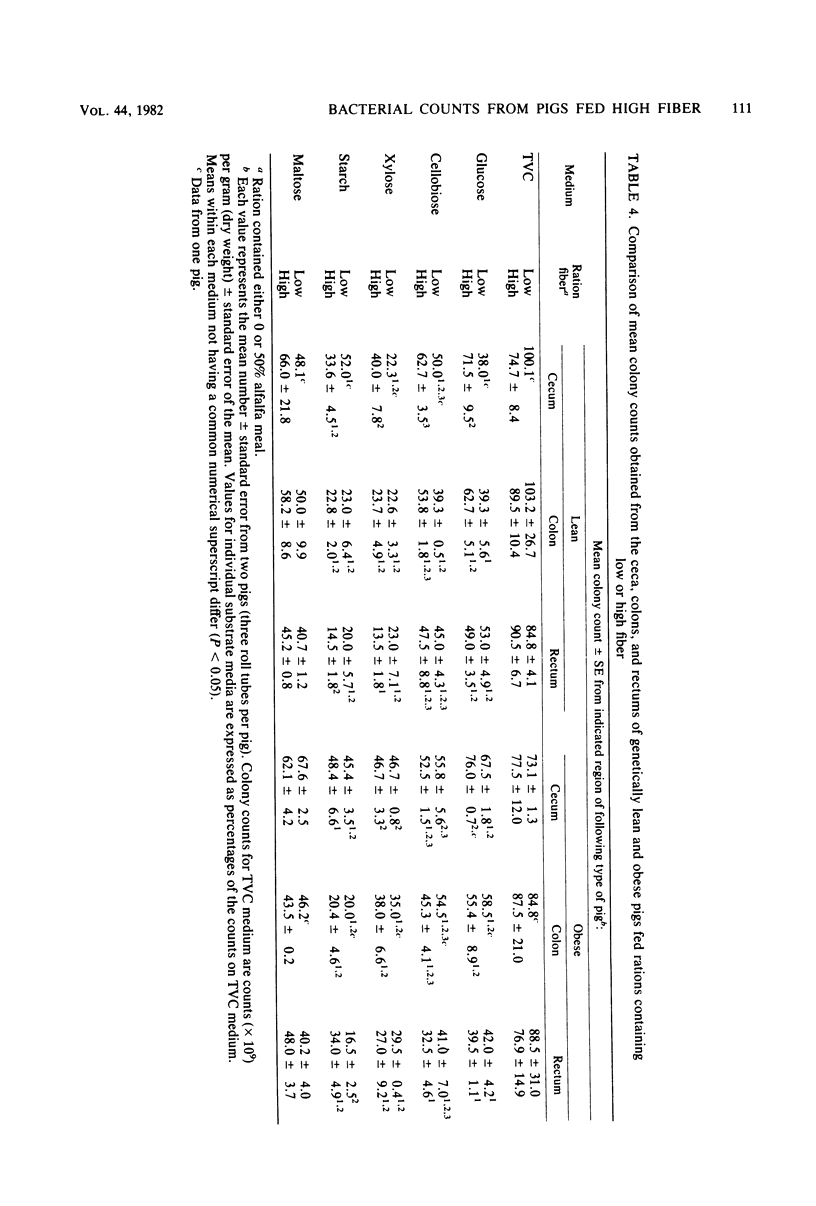
Abstract
Bacterial populations from gastrointestinal tracts of genetically lean and obese pigs fed a low- or high-fiber diet (0 or 50% alfalfa meal, respectively) were enumerated with rumen fluid media and specific energy sources. Total culture counts in rectal samples declined 56 (P greater than 0.05) and 63% (P less than 0.05) in lean and obese animals, respectively, 3 weeks after feeding the high-fiber diet. After 8 weeks, culture counts had risen and were similar to those obtained before alfalfa was fed (0 week). At slaughter, 12 to 17 weeks after feeding the high-fiber diet, total counts from rectal samples of lean pigs continued to rise and were 13% greater than the 0-week counts, whereas counts from obese animals declined 37% (P greater than 0.05). The number of cellulolytic bacteria in rectal samples of lean-genotype pigs fed the high-fiber diet increased 80 and 71% from 0 to 3 weeks and 3 to 8 weeks, respectively. This overall increases from 0 to 8 weeks in lean pigs was significant (P less than 0.05); however, these increases were not seen in obese pigs. These data suggest that the microflora is initially suppressed when exposed to a high-fiber diet and that later some adaptation takes place, apparently more so in lean than in obese pigs. When specific energy sources were used to delineate the distribution of different bacterial populations in the cecum, colon, and rectum, trends could be detected between high- and low-fiber diets. These data also support the concept that bacteria populations from different sites in the large bowel differ.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison M. J., Robinson I. M., Bucklin J. A., Booth G. D. Comparison of bacterial populations of the pig cecum and colon based upon enumeration with specific energy sources. Appl Environ Microbiol. 1979 Jun;37(6):1142–1151. doi: 10.1128/aem.37.6.1142-1151.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argenzio R. A., Southworth M. Sites of organic acid production and absorption in gastrointestinal tract of the pig. Am J Physiol. 1975 Feb;228(2):454–460. doi: 10.1152/ajplegacy.1975.228.2.454. [DOI] [PubMed] [Google Scholar]
- Betian H. G., Linehan B. A., Bryant M. P., Holdeman L. V. Isolation of a cellulotytic Bacteroides sp. from human feces. Appl Environ Microbiol. 1977 Apr;33(4):1009–1010. doi: 10.1128/aem.33.4.1009-1010.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- Dehority B. A., Grubb J. A. Basal medium for the selective enumeration of rumen bacteria utilizing specific energy sources. Appl Environ Microbiol. 1976 Nov;32(5):703–710. doi: 10.1128/aem.32.5.703-710.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leedle J. A., Hespell R. B. Differential carbohydrate media and anaerobic replica plating techniques in delineating carbohydrate-utilizing subgroups in rumen bacterial populations. Appl Environ Microbiol. 1980 Apr;39(4):709–719. doi: 10.1128/aem.39.4.709-719.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prynne C. J., Southgate D. A. The effects of a supplement of dietary fibre on faecal excretion by human subjects. Br J Nutr. 1979 May;41(3):495–503. doi: 10.1079/bjn19790064. [DOI] [PubMed] [Google Scholar]
- Robinson I. M., Allison M. J., Bucklin J. A. Characterization of the cecal bacteria of normal pigs. Appl Environ Microbiol. 1981 Apr;41(4):950–955. doi: 10.1128/aem.41.4.950-955.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell E. G. Types and distribution of anaerobic bacteria in the large intestine of pigs. Appl Environ Microbiol. 1979 Feb;37(2):187–193. doi: 10.1128/aem.37.2.187-193.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rérat A. Digestion and absorption of carbohydrates and nitrogenous matters in the hindgut of the omnivorous nonruminant animal. J Anim Sci. 1978 Jun;46(6):1808–1837. doi: 10.2527/jas1978.4661808x. [DOI] [PubMed] [Google Scholar]
- Salanitro J. P., Blake I. G., Muirhead P. A. Isolation and identification of fecal bacteria from adult swine. Appl Environ Microbiol. 1977 Jan;33(1):79–84. doi: 10.1128/aem.33.1.79-84.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavin J. L., Brauer P. M., Marlett J. A. Neutral detergent fiber, hemicellulose and cellulose digestibility in human subjects. J Nutr. 1981 Feb;111(2):287–297. doi: 10.1093/jn/111.2.287. [DOI] [PubMed] [Google Scholar]
- Varel V. H., Hashimoto A. G., Chen Y. R. Effect of temperature and retention time on methane production from beef cattle waste. Appl Environ Microbiol. 1980 Aug;40(2):217–222. doi: 10.1128/aem.40.2.217-222.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


