Abstract
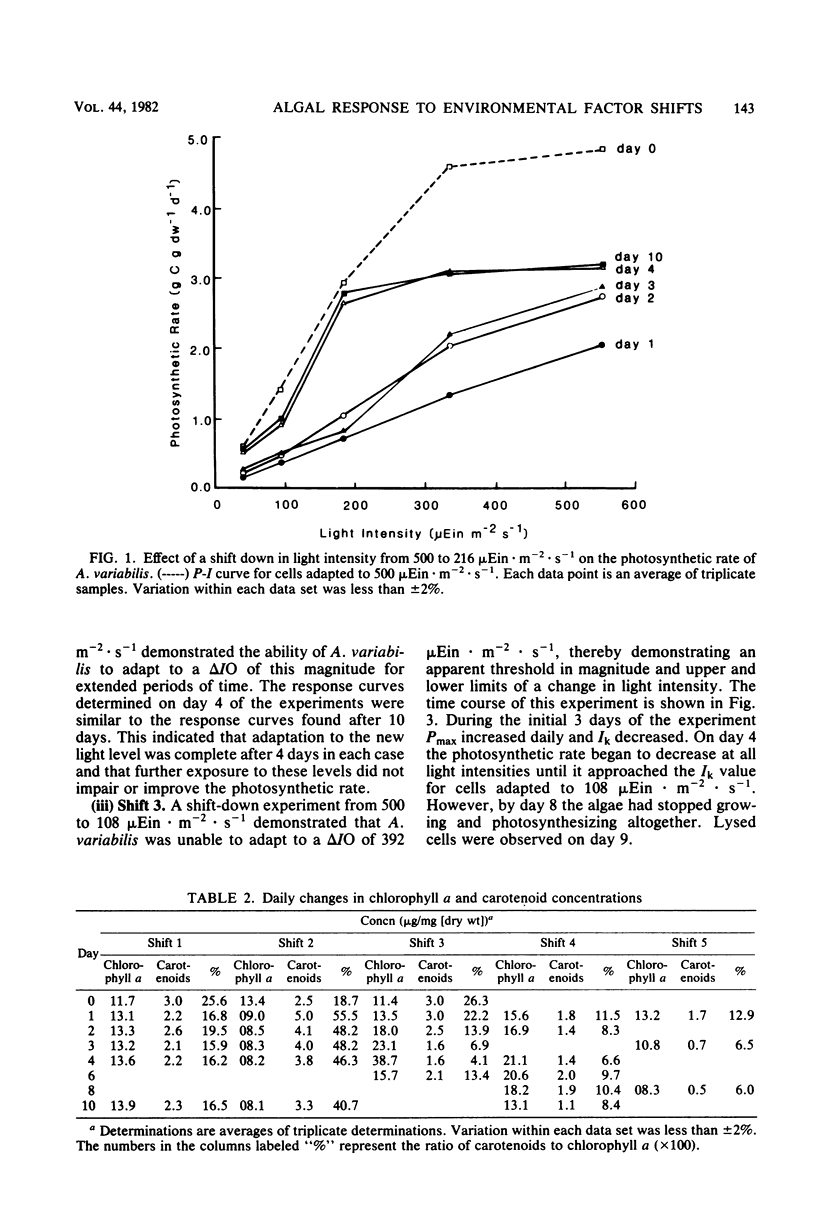
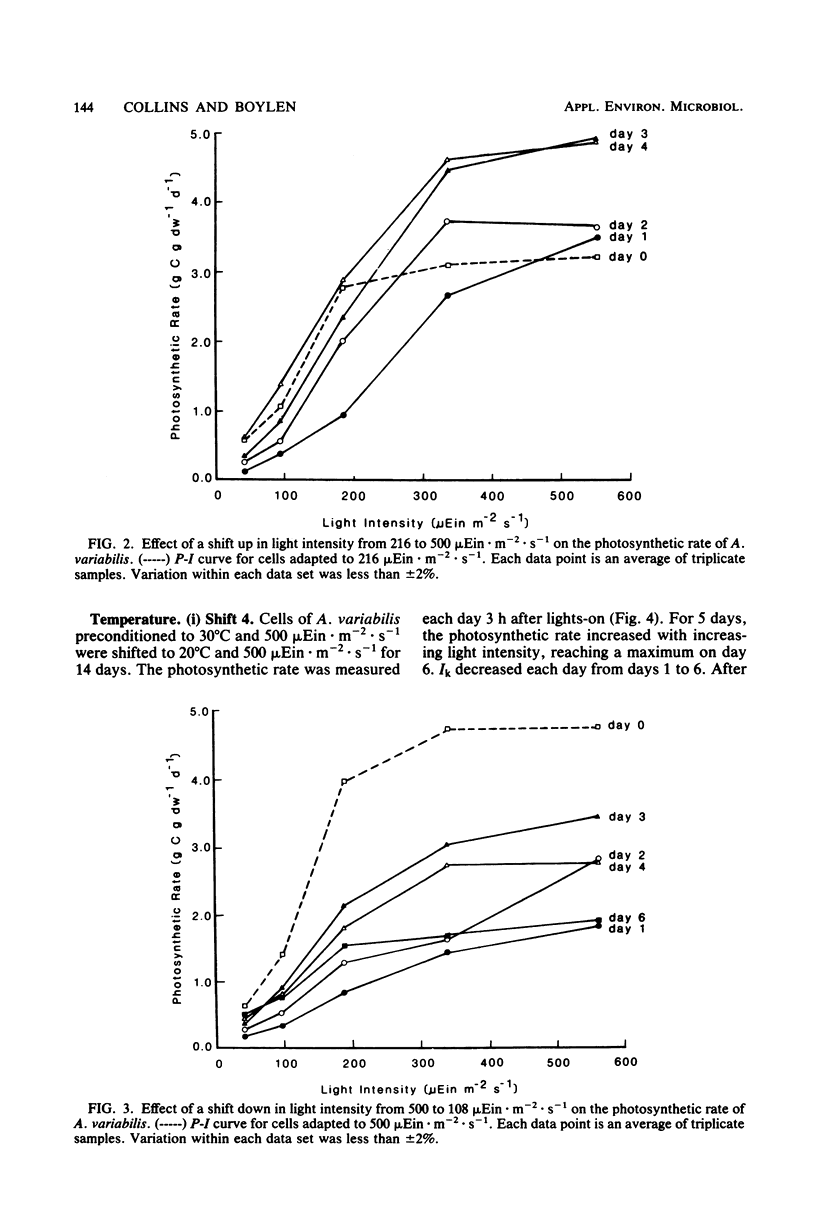
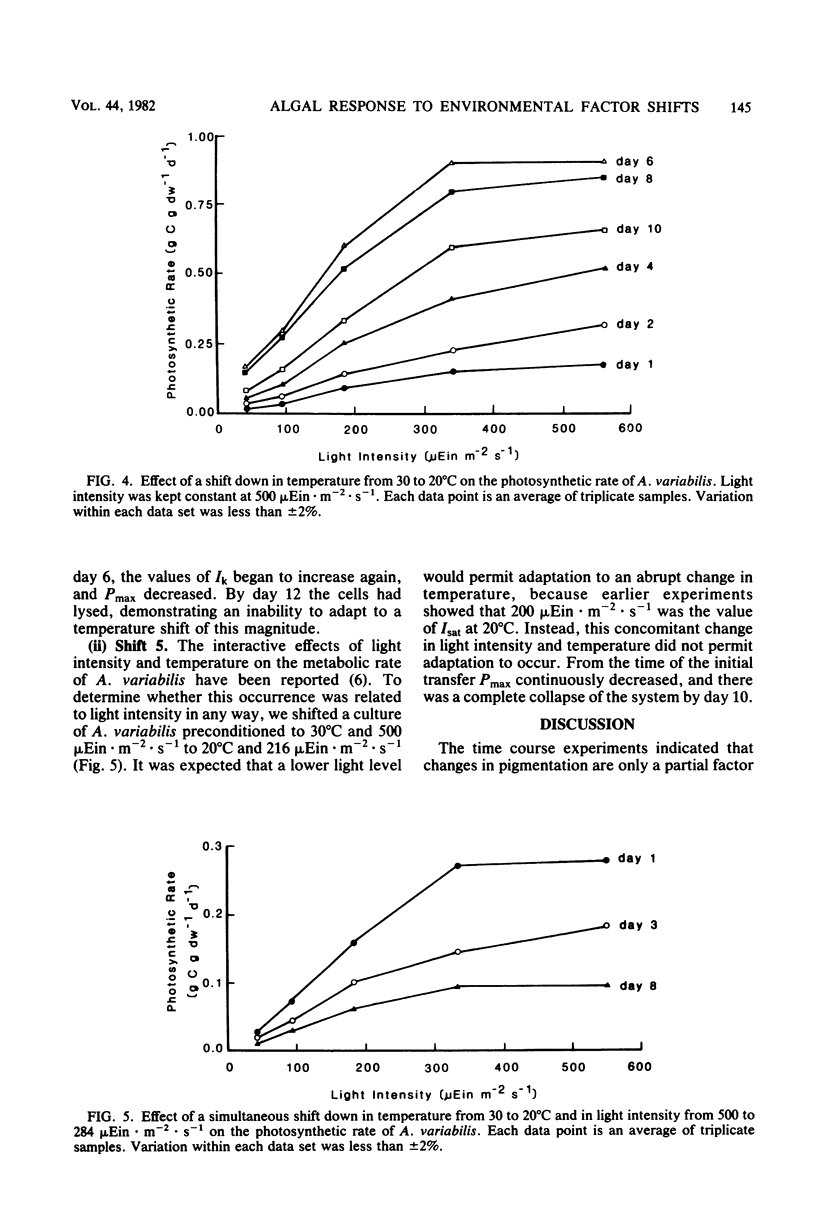
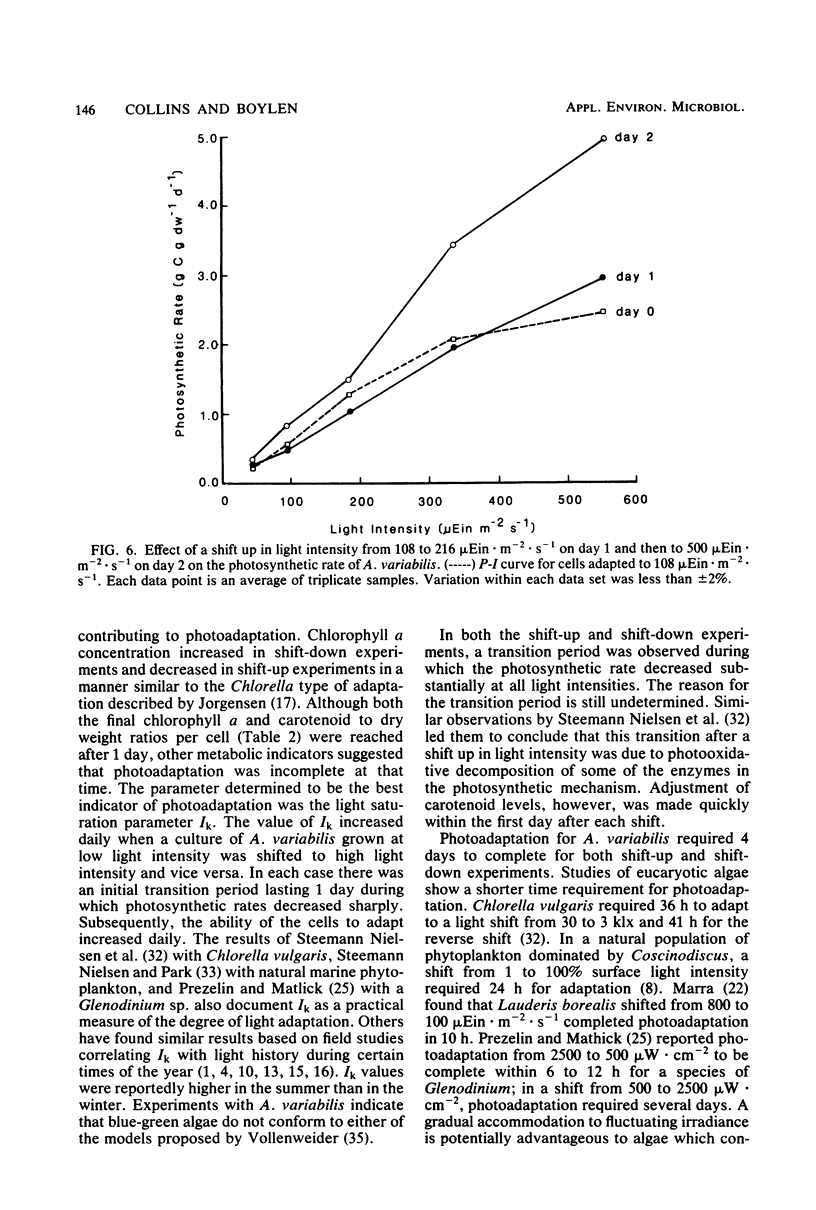
Cultures of Anabaena variabilis were exposed to different light intensities, and the time course of photoadaptation was measured by photosynthetic rate and changes in pigmentation. A shift down in intensity of 284 μEin · m−2 · sec−1 caused a temporary decrease in the photosynthetic response followed by gradual adaptation to the new conditions. Final chlorophyll a and carotenoid concentrations were reached after 1 day, although other physiological indicators showed that adaptation required 4 days. The parameter Ik was shown to be the best indicator of photoadaptation. A shift up in light intensity of the same magnitude also required 4 days for complete photoadaptation by the culture, although chlorophyll and carotenoid concentrations stabilized within 1 day. A shift down in light intensity of 392 μEin · m−2 · sec−1 resulted in a temporary attempt to adapt followed by collapse of the population. This demonstrates an apparent threshold in the magnitude of the shift in light intensity which will permit successful adaptation. Simultaneous changes in light intensity and temperature also adversely affected culture populations. Our observations present a possible cause for the decline or prevention of an algal bloom under a fluctuating light regime and suggest that it may be possible to predict this decline as a result of synoptic weather patterns or hydrodynamic influences.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Falkowski P. G., Owens T. G. Light-Shade Adaptation : TWO STRATEGIES IN MARINE PHYTOPLANKTON. Plant Physiol. 1980 Oct;66(4):592–595. doi: 10.1104/pp.66.4.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron H. A., Mauzerall D. The development of photosynthesis in a greening mutant of chlorella and an analysis of the light saturation curve. Plant Physiol. 1972 Jul;50(1):141–148. doi: 10.1104/pp.50.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellar P. E., Paerl H. W. Physiological adaptations in response to environmental stress during an n(2)-fixing anabaena bloom. Appl Environ Microbiol. 1980 Sep;40(3):587–595. doi: 10.1128/aem.40.3.587-595.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka A., Brock T. D. Changes in photosynthetic rate and pigment content of blue-green algae in Lake Mendota. Appl Environ Microbiol. 1978 Mar;35(3):527–532. doi: 10.1128/aem.35.3.527-532.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J., Graham J. R. The photosynthetic unit in chlorella measured by repetitive short flashes. Plant Physiol. 1971 Sep;48(3):282–286. doi: 10.1104/pp.48.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riper D. M., Owens T. G., Falkowski P. G. Chlorophyll Turnover in Skeletonema costatum, a Marine Plankton Diatom. Plant Physiol. 1979 Jul;64(1):49–54. doi: 10.1104/pp.64.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]


