Abstract
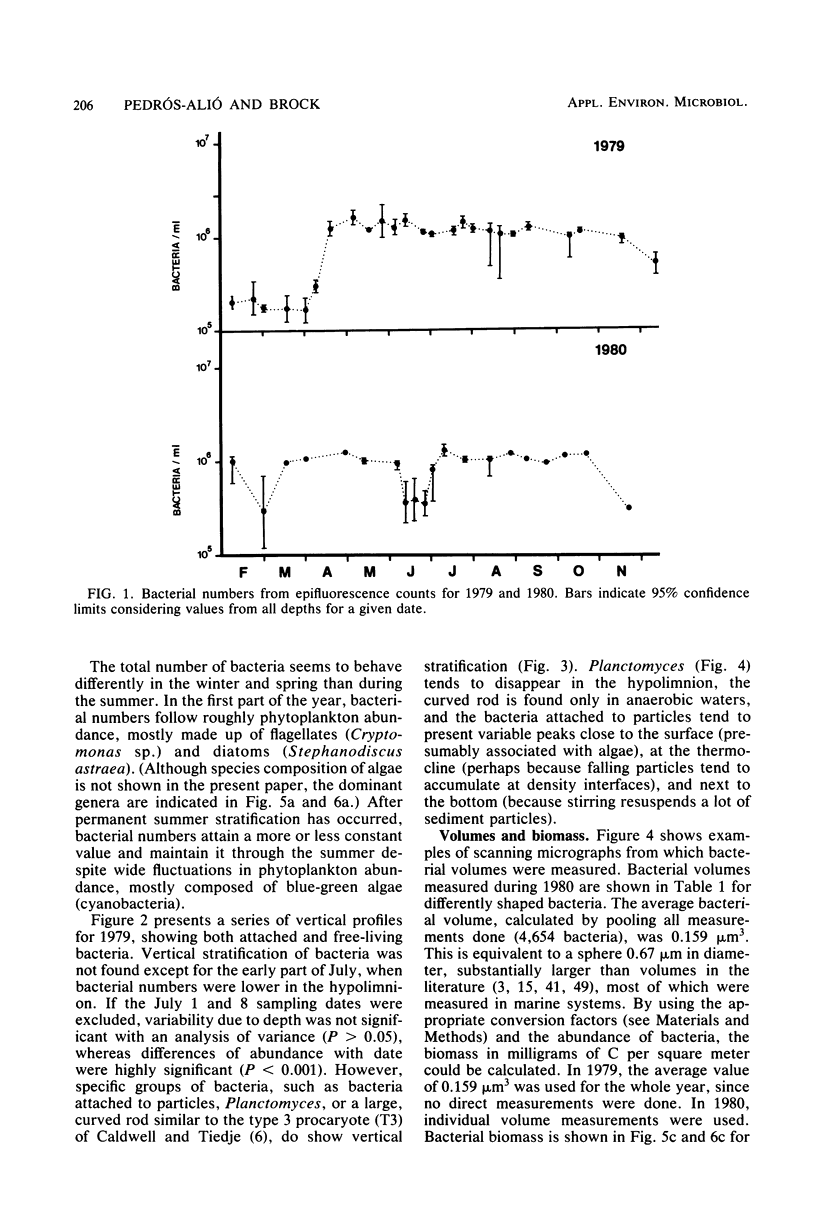
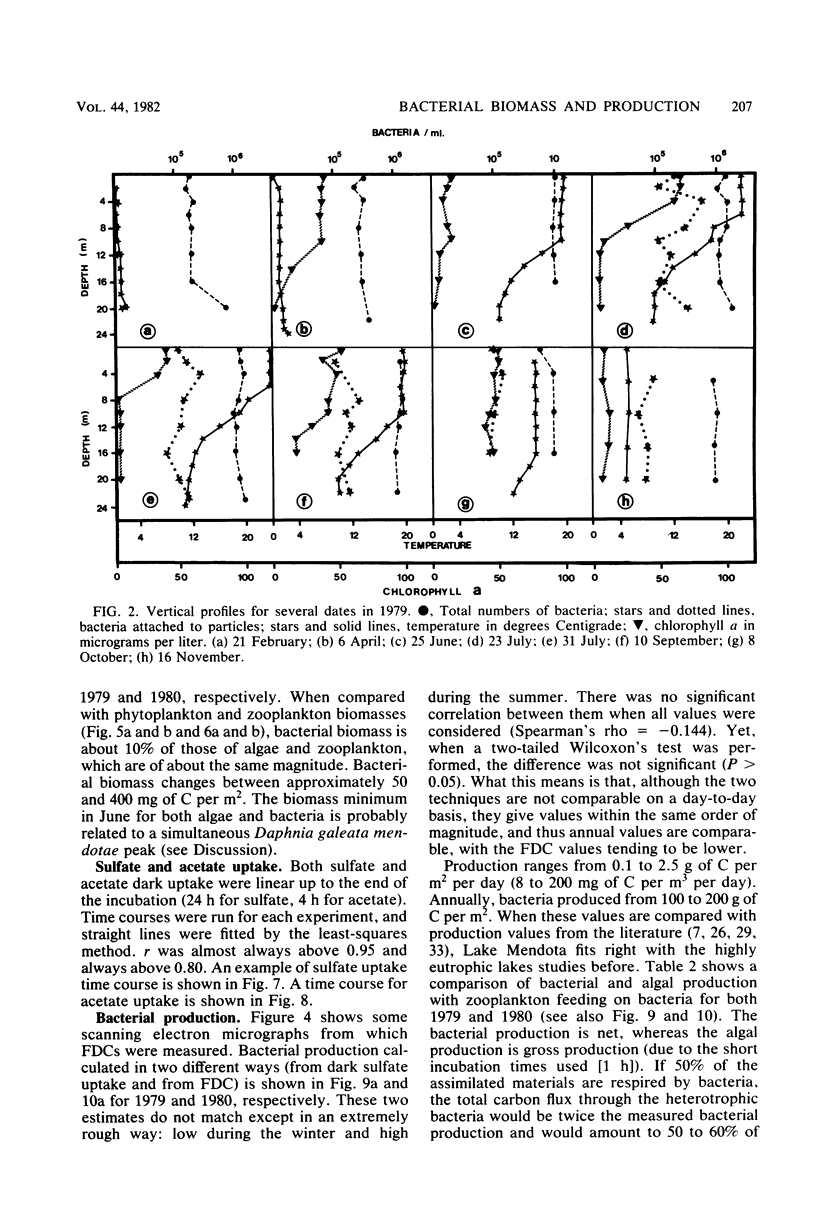
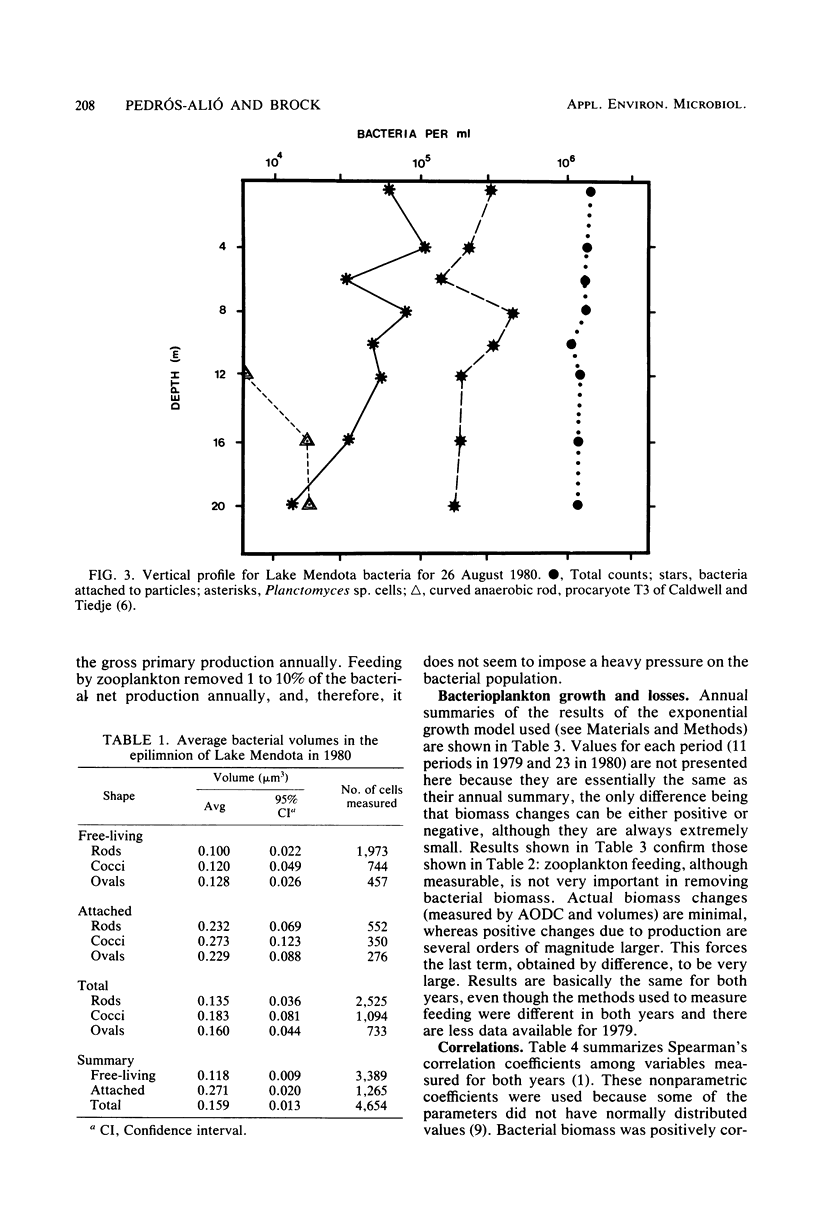
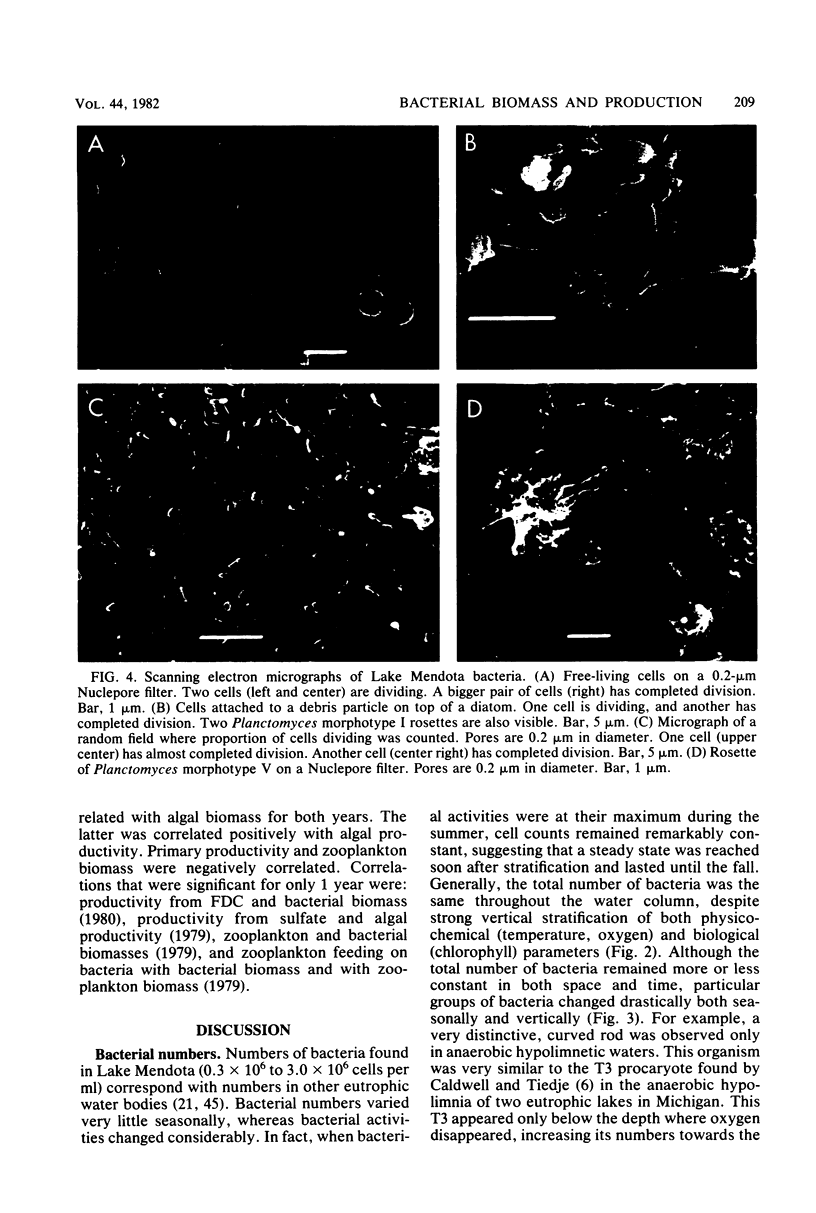
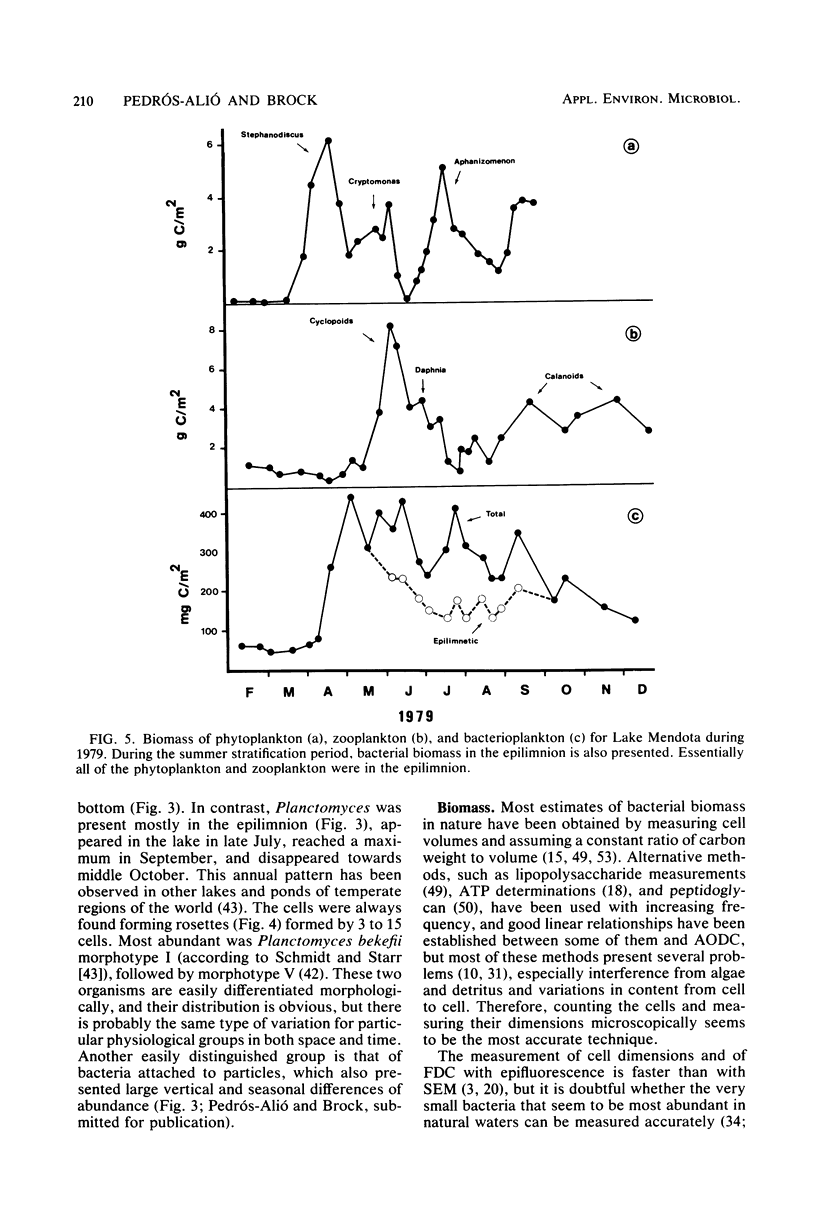
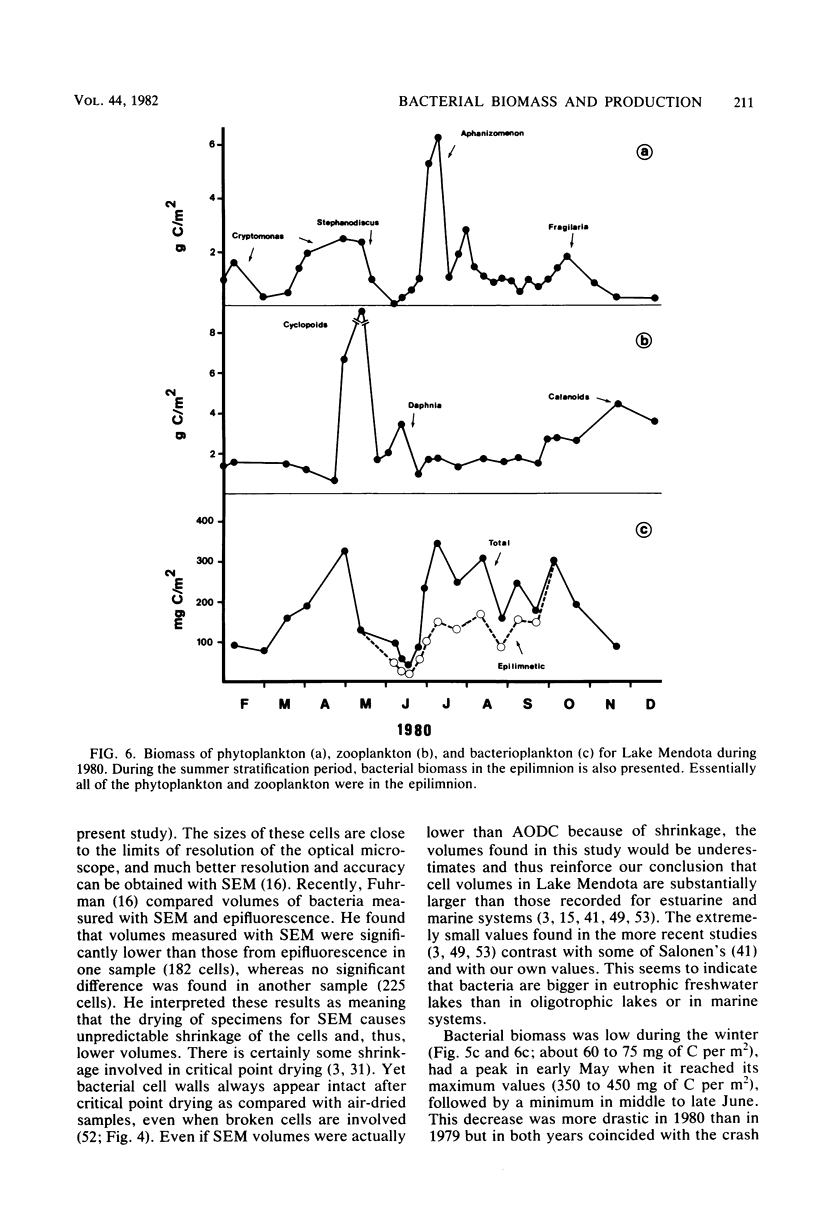
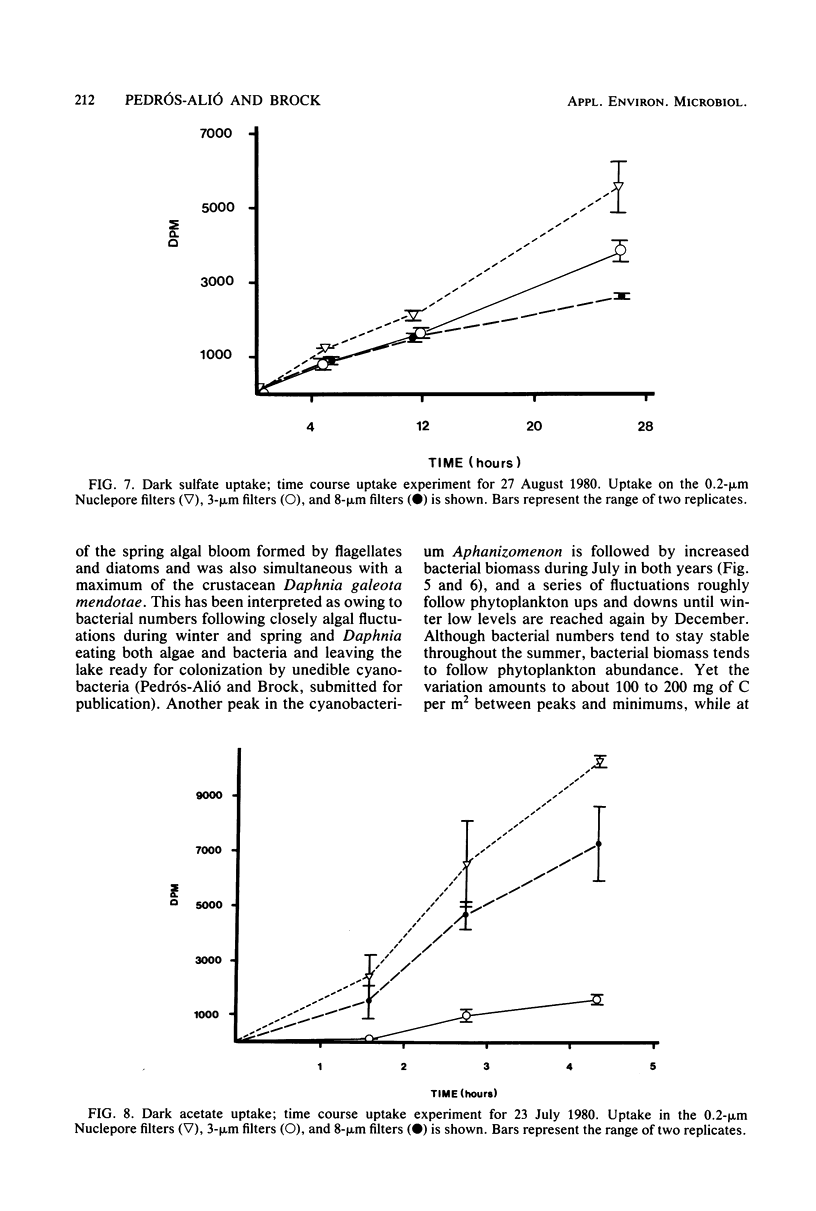
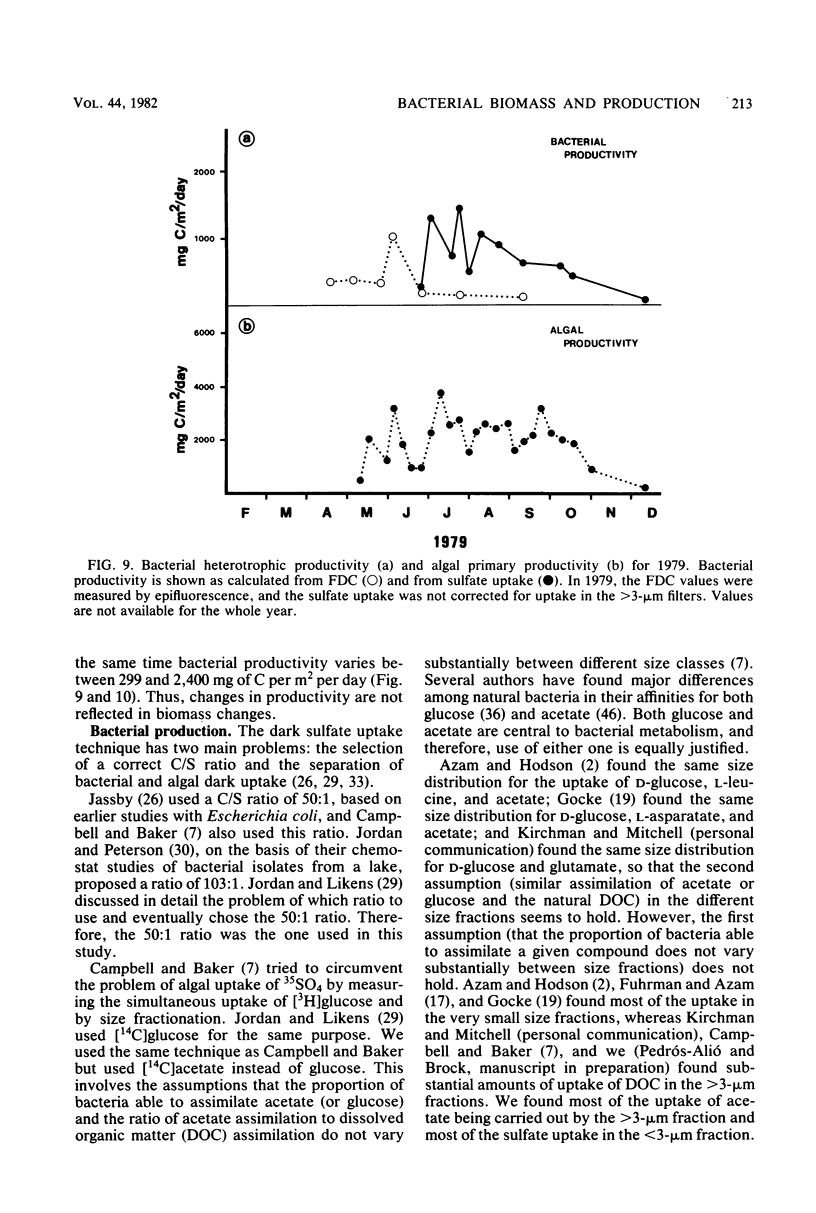
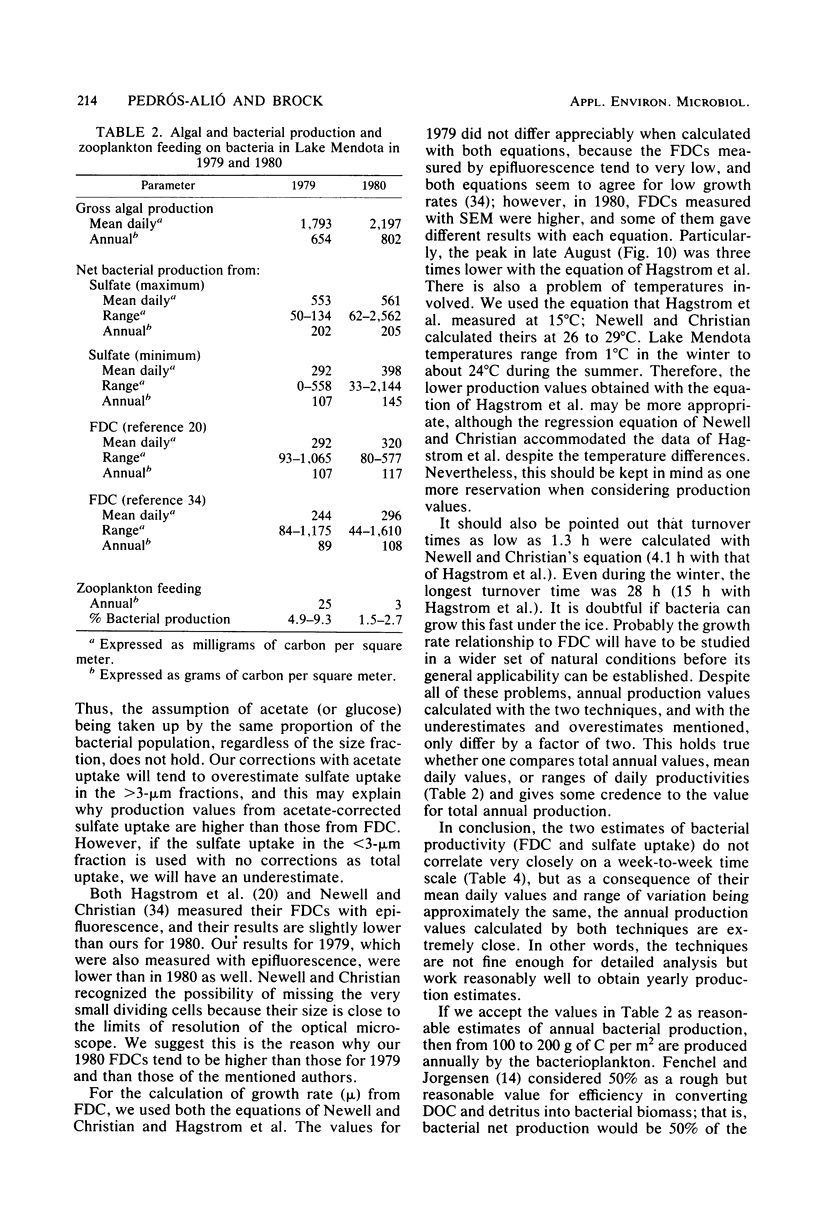
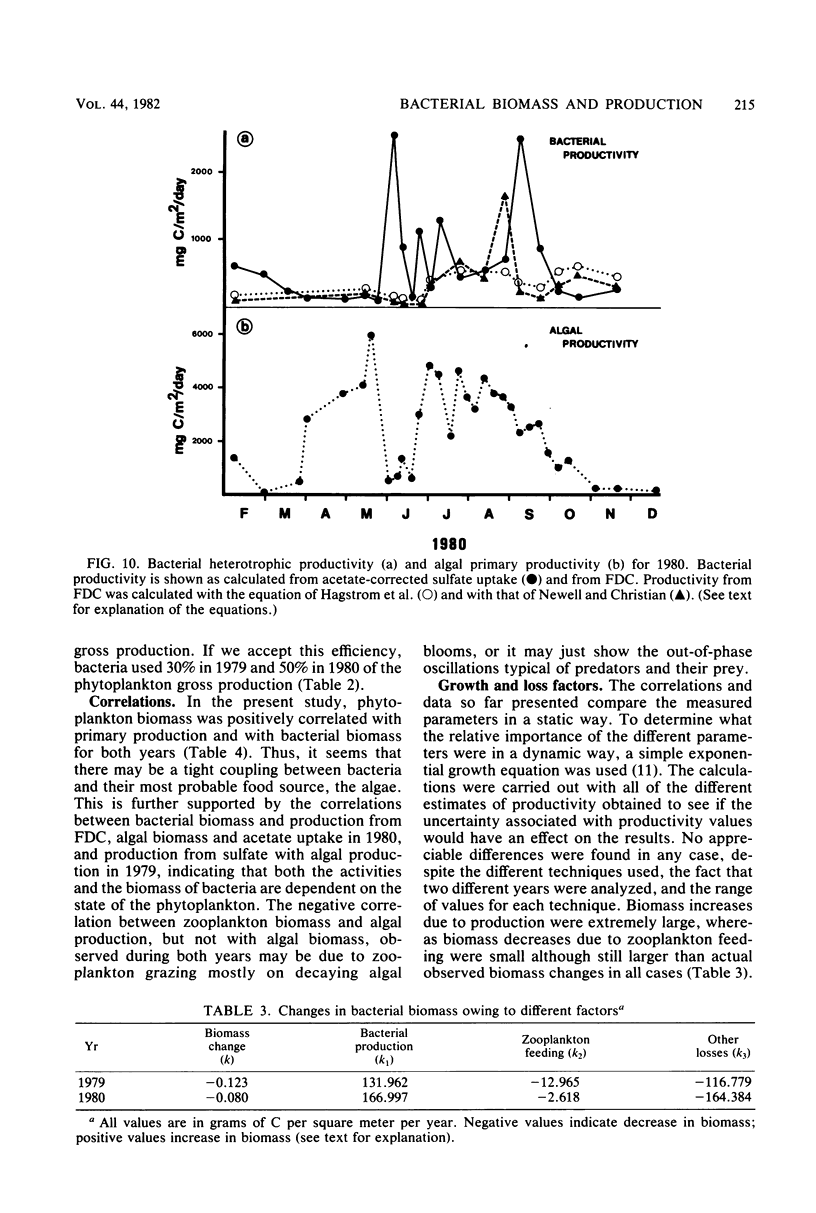
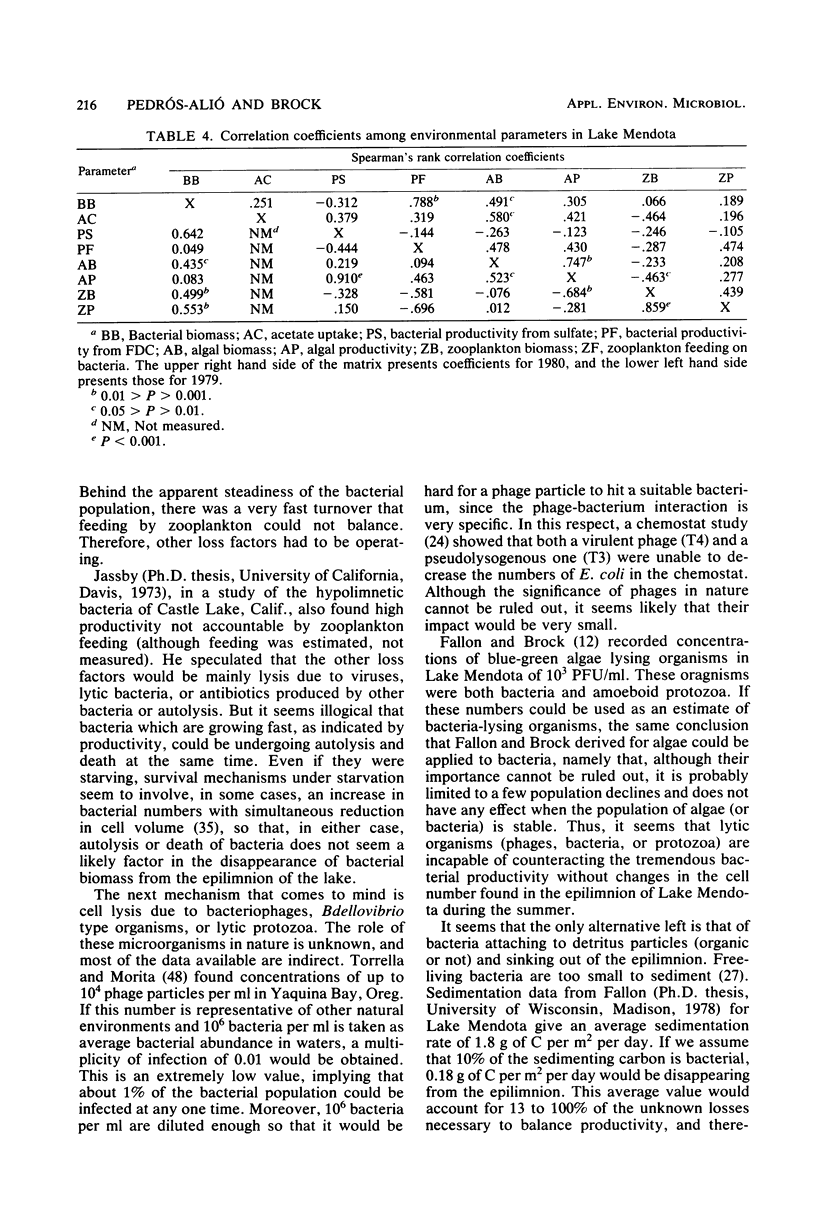
Estimates were made of the biomass and production of heterotrophic bacteria in the epilimnion of Lake Mendota, Wis. Cell counts were done with epifluorescence microscopy and varied from 3 × 105 bacteria per ml in winter to 3 × 106 bacteria per ml in summer. Cell volumes were measured in scanning electron micrographs. The average cell volume was 0.159 μm3. Annual variations and depth distribution were studied. Production was estimated from the frequency of dividing cells and from dark radioactive sulfate uptake. Annual productivity and daily average productivity were very close with both methods: 107 to 205 g of C per m2 per year for sulfate and 89 to 117 g of C per m2 per year for frequency of dividing cells. Zooplankton feeding removed 2 to 10% of the bacterial net production annually. When compared with biomass changes and losses due to zooplankton feeding, production values were very high. Therefore, it was suggested that other loss factors have to be more important than zooplankton feeding in controlling the bacterial population. Bacterial heterotrophic production was about 50% of gross primary production.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowden W. B. Comparison of two direct-count techniques for enumerating aquatic bacteria. Appl Environ Microbiol. 1977 May;33(5):1229–1232. doi: 10.1128/aem.33.5.1229-1232.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock T. D. Microbial growth rates in nature. Bacteriol Rev. 1971 Mar;35(1):39–58. doi: 10.1128/br.35.1.39-58.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell D. E., Tiedje J. M. A morphological study of anaerobic bacteria from the hypolimnia of two Michigan lakes. Can J Microbiol. 1975 Mar;21(3):362–376. doi: 10.1139/m75-051. [DOI] [PubMed] [Google Scholar]
- Campbell P. G., Baker J. H. Estimation of bacterial production in fresh waters by the simultaneous measurement of [35S]sulphate and d-[3H]glucose uptake in the dark. Can J Microbiol. 1978 Aug;24(8):939–946. doi: 10.1139/m78-156. [DOI] [PubMed] [Google Scholar]
- Fallon R. D., Brock T. D. Lytic organisms and photooxidative effects: influence on blue-green algae (cyanobacteria) in lake mendota, wisconsin. Appl Environ Microbiol. 1979 Sep;38(3):499–505. doi: 10.1128/aem.38.3.499-505.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman J. A., Azam F. Bacterioplankton secondary production estimates for coastal waters of british columbia, antarctica, and california. Appl Environ Microbiol. 1980 Jun;39(6):1085–1095. doi: 10.1128/aem.39.6.1085-1095.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagström A., Larsson U., Hörstedt P., Normark S. Frequency of dividing cells, a new approach to the determination of bacterial growth rates in aquatic environments. Appl Environ Microbiol. 1979 May;37(5):805–812. doi: 10.1128/aem.37.5.805-812.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne M. T. Coevolution of Escherichia coli and bacteriophages in chemostat culture. Science. 1970 May 22;168(3934):992–993. doi: 10.1126/science.168.3934.992-a. [DOI] [PubMed] [Google Scholar]
- Jannasch H. W. Estimations of bacterial growth rates in natural waters. J Bacteriol. 1969 Jul;99(1):156–160. doi: 10.1128/jb.99.1.156-160.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jassby A. D. The ecological significance of sinking to planktonic bacteria. Can J Microbiol. 1975 Mar;21(3):270–274. doi: 10.1139/m75-038. [DOI] [PubMed] [Google Scholar]
- Jordan M. J. On counseling minority students in a university center. J Am Coll Health Assoc. 1974 Dec;23(2):146–150. [PubMed] [Google Scholar]
- Krambeck C., Krambeck H. J., Overbeck J. Microcomputer-assisted biomass determination of plankton bacteria on scanning electron micrographs. Appl Environ Microbiol. 1981 Jul;42(1):142–149. doi: 10.1128/aem.42.1.142-149.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell S. Y., Christian R. R. Frequency of dividing cells as an estimator of bacterial productivity. Appl Environ Microbiol. 1981 Jul;42(1):23–31. doi: 10.1128/aem.42.1.23-31.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrella F., Morita R. Y. Evidence by electron micrographs for a high incidence of bacteriophage particles in the waters of Yaquina Bay, oregon: ecological and taxonomical implications. Appl Environ Microbiol. 1979 Apr;37(4):774–778. doi: 10.1128/aem.37.4.774-778.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. W., Novitsky T. J., Quinby H. L., Valois F. W. Determination of bacterial number and biomass in the marine environment. Appl Environ Microbiol. 1977 Apr;33(4):940–946. doi: 10.1128/aem.33.4.940-946.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]



