Abstract
In the mammalian retina, extensive processing of spatiotemporal and chromatic information occurs. One key principle in signal transfer through the retina is parallel processing. Two of these parallel pathways are the ON- and OFF-channels transmitting light and dark signals. This dual system is created in the outer plexiform layer, the first relay station in retinal signal transfer. Photoreceptors release glutamate onto ON- and OFF-type bipolar cells, which are functionally distinguished by their postsynaptic expression of different types of glutamate receptors, namely ionotropic and metabotropic glutamate receptors. In the current concept, rod photoreceptors connect only to rod bipolar cells (ON-type) and cone photoreceptors connect only to cone bipolar cells (ON- and OFF-type). We have studied the distribution of (RS)-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) glutamate receptor subunits at the synapses in the outer plexiform layer of the rodent retina by immunoelectron microscopy and serial section reconstruction. We report a non-classical synaptic contact and an alternative pathway for rod signals in the retina. Rod photoreceptors made synaptic contact with putative OFF-cone bipolar cells that expressed the AMPA glutamate receptor subunits GluR1 and GluR2 on their dendrites. Thus, in the retina of mouse and rat, an alternative pathway for rod signals exists, where rod photoreceptors bypass the rod bipolar cell and directly excite OFF-cone bipolar cells through an ionotropic sign-conserving AMPA glutamate receptor.
Complex processing of visual signals occurs in the retina. The anatomical basis is the multiple types of retinal neurons and their connections (1–5). The molecular basis is the different neurotransmitters and neuroactive substances released by the retinal neurons and the plethora of functionally diverse neurotransmitter receptors that mediate their effects (6–14). To understand retinal function or the function of any part of the nervous system, a detailed knowledge of transmitter receptors and their cellular and subcellular localization is required. An example is the parallel ON- and OFF-channels for the transmission of light and dark signals through the mammalian retina. Photoreceptors are depolarized in darkness and release glutamate, whereas in light they are hyperpolarized. In darkness OFF-bipolar cells are depolarized, whereas ON-bipolar cells are hyperpolarized. This dichotomy is created at the first retinal synapse, the synapse between photoreceptors and ON- and OFF-bipolar cells in the outer plexiform layer (OPL). The postsynaptic bipolar cells express functionally different types of glutamate receptors on their dendrites. The OFF-bipolar cells carry ionotropic glutamate receptors of the kainate/(RS)-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) type that sign conserve the photoreceptor signal, and the ON-bipolar cells carry a metabotropic glutamate receptor, mGluR6, that sign inverts the photoreceptor signal (9, 15–19). Although the mammalian cone photoreceptors connect to approximately 10 different types of ON- and OFF-cone bipolar cells, the rod photoreceptors are thought to connect to only one type of ON-rod bipolar cell (20–25). The classical pathway for rod signals in the mammalian retina is: rods → rod bipolar cells → AII amacrine cells → cone bipolar cells → ganglion cells. An alternative pathway for rod signals is the direct signal transmission from rods to cones via gap junctions and then to the inner retina via cone bipolar cells (26, 27). Recently it has been shown that a third pathway for rod signals exists. In this study, the authors recorded OFF-ganglion cell responses in a coneless retina of a transgenic mouse (28). Their findings suggested a synaptic contact between rod photoreceptors and cone bipolar cells.
In the search for the anatomical and molecular substrate of such an alternative rod-to-cone bipolar cell circuit, we have examined the localization of the AMPA glutamate receptor subunits at the synapses in the OPL of the rodent retina by immunoelectron microscopy and serial section reconstruction.
Materials and Methods
All experiments were performed in compliance with the guidelines for the welfare of experimental animals issued by the Federal Government of Germany, the National Institutes of Health, and the Max Planck Society.
Antibodies Used and Antibody Specificity.
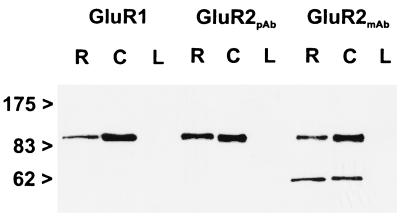
The following affinity-purified antibodies were used in this study: polyclonal anti-GluR1 antiserum (Chemicon), polyclonal anti-GluR2 antiserum (Chemicon), and monoclonal anti-GluR2 antibody (PharMingen). The antibodies against the different AMPA glutamate receptor subunits have been characterized and used previously for light and electron microscopic localization studies (12, 29, 30). In addition, we assessed the specificity of the antibodies by the immunoblotting of membrane proteins (5–10 μg/lane) of rat retina (R), cerebellum (C), and liver (L) (Fig. 1). The antibodies were used for immunoblotting and for light and electron microscopic immunocytochemistry at a concentration of 1.0 μg protein/ml for the polyclonal antisera against GluR1 and GluR2 and at a concentration of 5.0 μg protein/ml for the monoclonal antibody against GluR2. Each of the antibodies labeled a single band of protein in retina (Fig. 1, R) and cerebellum (Fig. 1, C) membrane preparations. The labeled proteins all had an apparent molecular mass of 100 kDa. For the monoclonal antibody against GluR2, an additional band in retina and cerebellum at an apparent molecular mass of about 60 kDa was detected. This band appears to be a breakdown product of GluR2. No band was detected in rat liver (Fig. 1, L).
Figure 1.
Immunoblots (7.5% gel SDS/PAGE) of proteins from rat retina (R), cerebellum (C), and liver (L) membrane preparations, probed with antibodies raised against the AMPA receptor subunits GluR1 and GluR2. Numbers and arrowheads (Left) indicate the position and relative molecular mass (kDa) of the standards. pAb, polyclonal antiserum; mAb, monoclonal antibody.
Tissue Preparation and Light and Electron Microscopic Immunocytochemistry of Mouse and Rat Retina Sections.
Retinae of adult albino mice and rats were investigated. The animals were anesthetized deeply with halothane and decapitated. The preparation of retinal tissue for light and electron microscopy and the immunostaining procedure were performed as described (31).
Briefly, for light microscopy, the tissue was fixed in 4% (wt/vol) paraformaldehyde in phosphate buffer (PB; 0.1 M, pH 7.4) for 15 to 30 min. The retinae were dissected free, cryoprotected in 10% (wt/vol), 20% (wt/vol) sucrose in PB for 1 hr each and in 30% (wt/vol) sucrose in PB overnight at 4°C. Pieces of retina were mounted in freezing medium (Reichert), sectioned vertically at 12-μm thickness on a cryostat, and collected on coated slides. Incubation time in the primary antibodies was overnight at room temperature. Immunocytochemical labeling was carried out by using the indirect fluorescence method. The binding sites of the primary antibodies were revealed by red fluorescing secondary antibodies goat anti-rabbit IgG and goat anti-mouse IgG (Alexa 594; Molecular Probes) diluted 1:500.
For electron microscopy, the tissue was fixed in 4% (wt/vol) paraformaldehyde in PB for 50 min. Because of the fixation sensitivity of the antibodies, no glutaraldehyde was used. After dissection and cryoprotection, the retinae were frozen and thawed repeatedly to enhance the penetration of the antibodies. Small pieces of retina were embedded in agar, and vertical sections (50-μm thick) were cut with a vibratome for preembedding electron microscopic immunocytochemistry. The primary antibodies were used at the same concentration and diluted in the same medium, but without Triton X-100, as used for light microscopy. Incubation time in the primary antibodies was 4 d at 4°C. The binding sites of the primary antibodies were visualized by peroxidase staining followed by silver intensification and gold toning (31). Ultrathin sections were cut and stained with uranyl aceteate and lead citrate.
Microscopic Analysis.
For light microscopic analysis, the sections were examined and photographed with a Zeiss photomicroscope (Axiophot, Zeiss) by using ×40 and ×63 objectives and the appropriate fluorescence filters (Cy3/Texas red: BP 546, FT 580, LP 590). Black-and-white photomicrographs were taken on Kodak TMY 400 film. For electron microscopic analysis, ultrathin sections were examined and photographed with a Zeiss EM10 electron microscope. For serial section reconstruction of individual synapses, a GATAN BioScan digital camera (1,024 × 1,024 pixel; GATAN, Munich, Germany) in combination with the software program digital micrograph 3.1 (GATAN, Coronado Lane, Pleasanton, CA) was used.
Results
In vertical sections of adult mouse and rat retina, the antibodies against the AMPA receptor subunits GluR1 and GluR2 produced characteristic patterns of labeling in the outer and the inner plexiform layer. In the following, the localization of GluR1 and GluR2 at the synapses in the OPL will be described and discussed in detail.
GluR1 and GluR2 Are Present in the OPL.
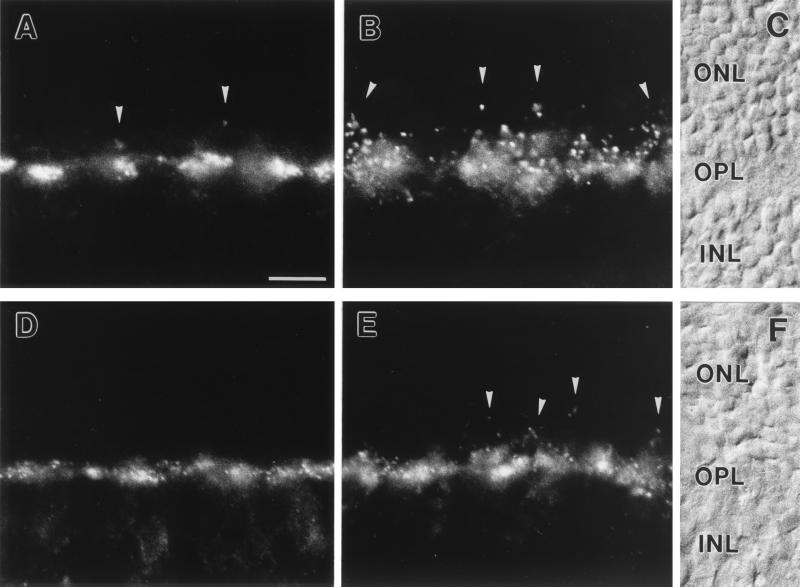
Light microscopic staining for both receptor subunits was strong in the OPL of mouse and rat retina, with a mixture of punctate and diffuse appearances (Fig. 2). From the pattern of the labeling, GluR1 seemed to be expressed on neuronal processes associated with the terminals of cone photoreceptors (Fig. 2 A and D), whereas GluR2 seemed to be expressed on neuronal processes associated with the terminals of both cone and rod photoreceptors (Fig. 2 B and E). In addition, the strength of the immunosignals for GluR1 and GluR2 in the OPL differed between mouse (Fig. 2 A and B) and rat (Fig. 2 D and E), with stronger expression of the two subunits in the OPL of mouse.
Figure 2.
(A–C) Micrographs of vertical cryostat sections of mouse retina stained with an antiserum against GluR1 (A) and against GluR2 (B). (C) Nomarski micrograph showing the retinal layers (ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer). (D–F) Micrographs of vertical cryostat sections of rat retina stained with an antiserum against GluR1 (D) and against GluR2 (E). (F) Nomarski micrograph showing the retinal layers. For both species and for both glutamate receptor subunits, strong immunofluorescence was found in the OPL. The labeling was mainly associated with cone pedicles (the large clusters) but was also found at rod spherules (arrowheads). Bar = 10 μm in A for A–F.
GluR1 and GluR2 Are Present on Dendrites of OFF-Cone Bipolar Cells.
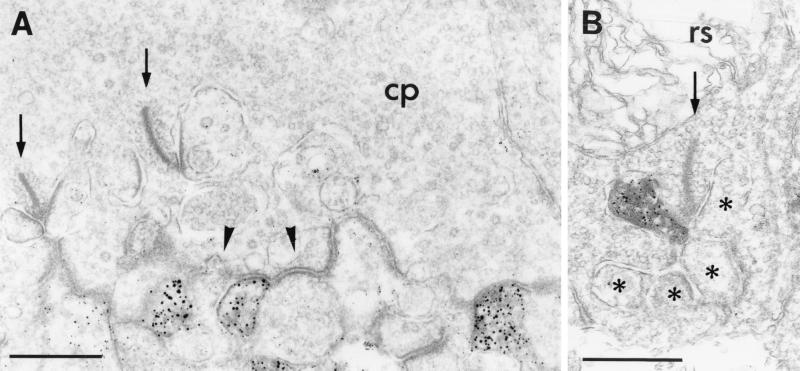
We used the highly sensitive method of preembedding immunocytochemistry to examine by electron microscopy the subcellular distribution of GluR1 and GluR2 at the synapses in the OPL. Immunoreactivity for both glutamate receptor subunits was found on the dendrites of OFF-cone bipolar cells, making flat noninvaginating synaptic contacts at cone pedicles (Fig. 3A). GluR2, but not GluR1, immunoreactivity was also frequently found on postsynaptic horizontal cell processes at both rod and cone synapses (Fig. 3B). In none of the many hundreds of cone and rod photoreceptor synapses examined was GluR1 or GluR2 immunoreactivity observed on the dendrites of rod bipolar cells (ON-type) or on the dendrites of invaginating cone bipolar cells (ON-type).
Figure 3.
(A) Electron micrograph showing the postsynaptic localization of GluR1 at a cone pedicle (cp) in the OPL of mouse retina. GluR1 immunoreactivity was found on some dendrites of OFF-cone bipolar cells making flat noninvaginating synaptic contacts at the cone pedicle; other flat contacts were not labeled (arrowhead). (B) Electron micrograph showing the localization of GluR2 on a horizontal cell process postsynaptic at a rod spherule (rs) in the OPL of rat retina. The presynaptic ribbons in the cone pedicle and in the rod spherule are marked by arrows; the unlabeled postsynaptic elements at the rod spherule are marked by asterisks. Bar = 0.5 μm in A and B.
A Non-Classical Synaptic Contact at Terminals of Rod Photoreceptors.
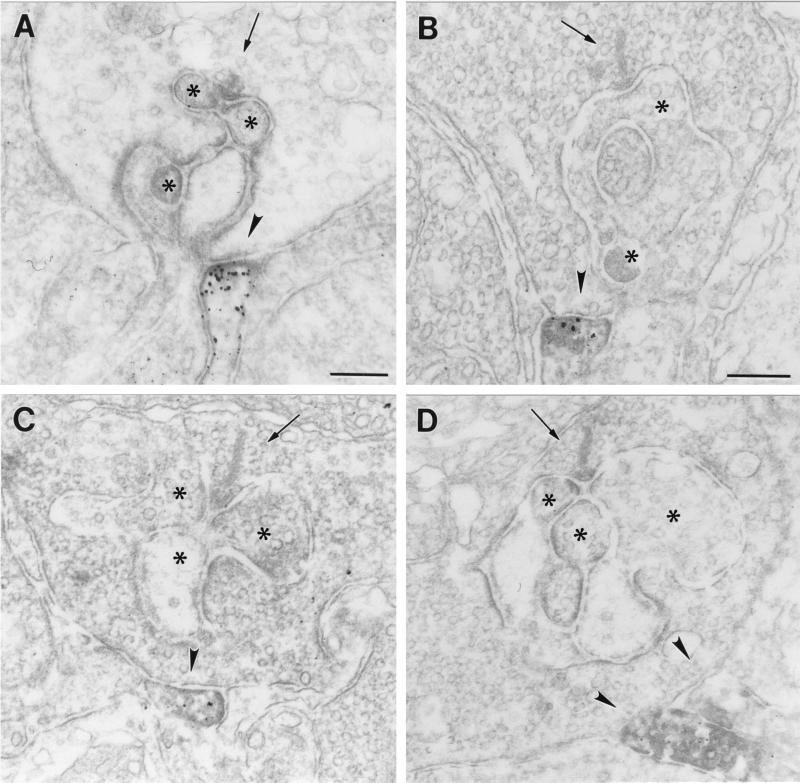
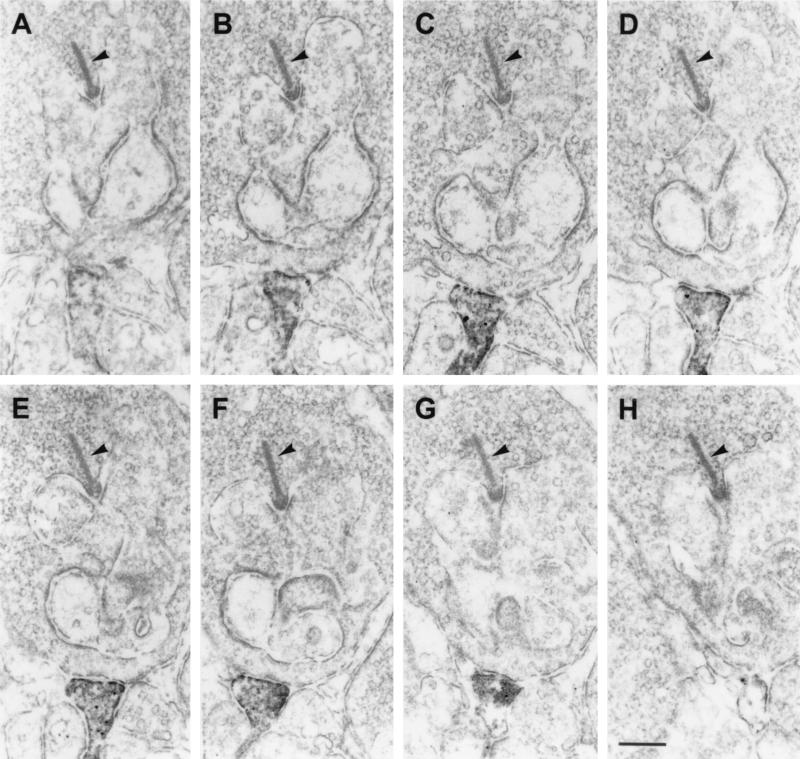
The most striking finding of this study was the presence of GluR1 and GluR2 immunoreactivity on dendritic processes, which made flat noninvaginating synaptic contacts at terminals of rod photoreceptors (Fig. 4). The ultrastructural appearance of these contacts is reminiscent of the synaptic contacts made by OFF-cone bipolar cell dendrites at cone pedicles (Fig. 3A). To unambiguously demonstrate that these processes are dendrites of putative OFF-cone bipolar cells, and that they do not invaginate into the rod terminals becoming a horizontal cell process or a rod bipolar cell dendrite, we performed serial sectioning and reconstruction of these synaptic contacts at rod terminals. In the example shown in Fig. 5, a process labeled for the receptor subunit GluR2 approaches the rod terminal and forms a flat basal contact. Over several sections, the staining gets stronger (Fig. 5 A–F), then weaker, and finally the process vanishes from the site of the rod terminal (Fig. 5 G and H). Throughout the series, the process never invaginates into the rod terminal. The number of such synaptic contacts of putative OFF-cone bipolar cell dendrites found at rod terminals was low (less than 5% of the examined rod photoreceptor terminals in mouse and rat retina), but one has to keep in mind that electron microscopic staining techniques tend to understain the tissue, and thus the 5% value is likely to be an underestimate.
Figure 4.
(A–D) Electron micrographs showing flat noninvaginating synaptic contacts (arrowhead) made by putative OFF-cone bipolar cell dendrites at rod spherules in the OPL of mouse (A and C) and rat (B and D) retina labeled for GluR1 (A and B) and for GluR2 (C and D). The presynaptic ribbons in the rod spherules are marked by an arrow. The unstained horizontal cell processes and dendrites of rod bipolar cells postsynaptic at the rod synapses are marked by asterisks. Bar = 0.2 μm in A for A, C, and D, and 0.2 μm in B.
Figure 5.
(A–H) Electron micrographs showing a series of ultrathin sections taken through a flat noninvaginating synaptic contact made by a putative OFF-cone bipolar cell dendrite labeled for GluR2 at a rod spherule in the OPL of rat. The presynaptic ribbon in the rod spherule is marked by an arrowhead. Bar = 0.2 μm.
Discussion
The ON- and OFF-channels in the mammalian retina are generated by cone photoreceptors connecting to several subtypes of ON- and OFF-cone bipolar cells and by rod photoreceptors connecting to one type of ON-rod bipolar cell. The ON- and OFF-type bipolar cells express functionally different types of glutamate receptors. Recent studies on the localization of the various known glutamate receptors in the OPL revealed an unexpectedly high degree of complexity. The effects of glutamate released by the photoreceptors are mediated and modulated by glutamate receptors, which are present post- and presynaptically and extrasynaptically at the photoreceptor synapses (9, 14, 19, 32–35).
It has also been reported that rod signals can reach ganglion cells over a pathway independent of the rod bipolar cells and the AII amacrine cells. This alternative circuit involves gap-junction coupling between rods and cones in the outer retina and is thought to subserve twilight vision (27, 36–39). Recently Soucy et al. (28), however, have shown that rod signals can reach OFF-ganglion cells in the retina of a coneless transgenic mouse. Because these signals could not take the route from rods to cones via gap junctions, and because transmission between the photoreceptors and the ON-bipolar cells was blocked by l-2-amino-4-phosphonobutyrate acid, this study suggested a synaptic link between rod photoreceptors and an unidentified type of OFF-cone bipolar cell.
We have examined the ultrastructural localization of AMPA glutamate receptor subunits at the synapses in the OPL of mouse and rat retina, and we think we have found the anatomical and molecular substrate for an alternative circuit for rod signals in the rodent retina as proposed by Soucy et al. (28). We describe a synaptic contact between rod photoreceptors and putative OFF-cone bipolar cells. The putative OFF-cone bipolar cells making flat noninvaginating contacts at the rod terminals expressed the glutamate receptor subunits GluR1 and GluR2 on their dendrites. We believe that these dendrites belong to OFF-cone bipolar cells, because the ultrastructural appearance of the contacts is similar to basal synapses made by OFF-cone bipolar cells at cone terminals, and because in a few cases a process seemed to contact both a cone and a rod terminal.
The AMPA glutamate receptor subunits GluR1 and GluR2 present on the dendrites can form functional ionotropic AMPA glutamate receptors (40). Thus, the rod signal is mediated through a sign-conserving AMPA glutamate receptor channel to putative OFF-cone bipolar cells, bypassing the classical rod pathway with the rod bipolar cell and the AII amacrine cell (Fig. 6). The main conclusion of the study of Soucy et al. (28) derives from the investigation of a transgenic mouse. Thus it is particularly noteworthy that our observations were made in wild-type mouse and rat, demonstrating that the pathway is a constituent of the normal retina.
Figure 6.
Schematic drawing of the alternative pathway for rod signals through the rodent retina. The OFF-pathway is shaded. Rod photoreceptors (R) bypass the rod bipolar cell (RB) and directly excite OFF-cone bipolar cells (CB). The signals are mediated by the AMPA glutamate receptor subunits GluR1 and GluR2 expressed postsynaptically on the dendrites of the OFF-cone bipolar cells. A, amacrine cell; G, ganglion cell.
In the retinae of cold-blooded vertebrates, it is common that rods and cones are connected to the same bipolar cell (24). In the rodent retina, we found a small percentage of rods contacting putative OFF-cone bipolar cells. The functional significance of such a synaptic link between rods and OFF-cone bipolar cells in the mammalian retina remains an open question, as is the question whether such a pathway is common to the mammalian retina or is a residue of evolution found only in the rodent retina. This question will have to be answered by comparative studies. In fact, there is evidence for bipolar cells making flat contacts on both rods and cones in the retina of the gray squirrel (41). West (41) showed that the bipolar cell in question clearly ramified in sublamina a of the retina, the OFF-layer, and thus was an OFF-cone bipolar cell. In agreement with Peter Sterling (42), we are convinced that not all second-order circuits are identified in the mammalian retina, and that the retina will provide many more surprises in the time to come.
Acknowledgments
We thank A. Hildebrand, H. Ahmed, W. Hofer, G. S. Nam, and D. Benzaid for excellent technical assistance and A. Hirano for reading and improving the manuscript. Special thanks to H. Wässle for his continuous support and input. This study was supported by a grant from the Deutsche Forschungsgemeinschaft (SFB 269/B4) and by a Heisenberg Fellowship to J.H.B.
Abbreviations
- AMPA
(RS)-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- GluR
glutamate receptor
- OPL
outer plexiform layer
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Dowling J E, Boycott B B. Proc R Soc London Ser B. 1966;166:80–111. doi: 10.1098/rspb.1966.0086. [DOI] [PubMed] [Google Scholar]
- 2.Boycott B B, Dowling J E. Philos Trans R Soc London B. 1969;799:109–194. [Google Scholar]
- 3.Wässle H, Boycott B B. Physiol Rev. 1991;71:447–480. doi: 10.1152/physrev.1991.71.2.447. [DOI] [PubMed] [Google Scholar]
- 4.MacNeil M A, Masland R H. Neuron. 1998;20:971–982. doi: 10.1016/s0896-6273(00)80478-x. [DOI] [PubMed] [Google Scholar]
- 5.Sterling P. In: The Synaptic Organization of the Brain. Shepherd G M, editor. New York: Oxford Univ. Press; 1998. pp. 205–253. [Google Scholar]
- 6.Yazulla S. In: Progress in Retinal Research. Osborne N N, Chader G J, editors. Oxford: Pergamon; 1986. pp. 1–52. [Google Scholar]
- 7.Marc R E. In: Progress in Retinal Research. Osborne N N, Chader G J, editors. Oxford: Pergamon; 1989. pp. 67–107. [Google Scholar]
- 8.Massey S M. In: Progress in Retinal Research. Osborne N N, Chader G J, editors. Oxford: Pergamon; 1990. pp. 399–425. [Google Scholar]
- 9.Masu M, Iwakabe H, Tagawa Y, Miyoshi T, Yamashita M, Fukuda Y, Sasaki H, Hiroi K, Nakamura Y, Shigemoto R, et al. Cell. 1995;80:757–765. doi: 10.1016/0092-8674(95)90354-2. [DOI] [PubMed] [Google Scholar]
- 10.Shiells R. In: Neurobiology and Clinical Aspects of the Outer Retina. Djamgoz M B A, Archer S N, Vallerga S, editors. London: Chapman & Hall; 1995. pp. 297–324. [Google Scholar]
- 11.Brandstätter J H, Koulen P, Wässle H. Vision Res. 1998;38:1385–1397. doi: 10.1016/s0042-6989(97)00176-4. [DOI] [PubMed] [Google Scholar]
- 12.Vardi N, Morigiwa K, Wang T-L, Shi Y-J, Sterling P. Vision Res. 1998;38:1359–1369. doi: 10.1016/s0042-6989(98)00007-8. [DOI] [PubMed] [Google Scholar]
- 13.Wässle H, Koulen P, Brandstätter J H, Fletcher E L, Becker C-M. Vision Res. 1998;38:1411–1430. doi: 10.1016/s0042-6989(97)00300-3. [DOI] [PubMed] [Google Scholar]
- 14.Quin P, Pourcho R G. Visual Neurosci. 1999;16:169–177. doi: 10.1017/s0952523899161121. [DOI] [PubMed] [Google Scholar]
- 15.Slaughter M M, Miller R F. Science. 1981;211:182–185. doi: 10.1126/science.6255566. [DOI] [PubMed] [Google Scholar]
- 16.Nawy S, Jahr C E. Nature (London) 1990;346:269–271. doi: 10.1038/346269a0. [DOI] [PubMed] [Google Scholar]
- 17.Euler T, Schneider H, Wässle H. J Neurosci. 1996;16:2934–2944. doi: 10.1523/JNEUROSCI.16-09-02934.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartveit E. J Neurophysiol. 1996;76:401–422. doi: 10.1152/jn.1996.76.1.401. [DOI] [PubMed] [Google Scholar]
- 19.Brandstätter J H, Koulen P, Wässle H. J Neurosci. 1997;17:9298–9307. doi: 10.1523/JNEUROSCI.17-23-09298.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolb H, Famiglietti E V. Science. 1974;186:47–49. doi: 10.1126/science.186.4158.47. [DOI] [PubMed] [Google Scholar]
- 21.Dacheux R F, Raviola E. J Neurosci. 1986;6:331–345. doi: 10.1523/JNEUROSCI.06-02-00331.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wässle H, Yamashita M, Greferath U, Grünert U, Müller F. Visual Neurosci. 1991;7:99–112. doi: 10.1017/s095252380001097x. [DOI] [PubMed] [Google Scholar]
- 23.Euler T, Wässle H. J Comp Neurol. 1995;361:461–478. doi: 10.1002/cne.903610310. [DOI] [PubMed] [Google Scholar]
- 24.Kolb H, Nelson R. In: Neurobiology and Clinical Aspects of the Outer Retina. Djamgoz M B A, Archer S N, Vallerga S, editors. London: Chapman & Hall; 1995. pp. 273–296. [Google Scholar]
- 25.Sterling P, Smith R G, Rao R, Vardi N. In: Neurobiology and Clinical Aspects of the Outer Retina. Djamgoz M B A, Archer S N, Vallerga S, editors. London: Chapman & Hall; 1995. pp. 325–348. [Google Scholar]
- 26.Smith R G, Freed M A, Sterling P. J Neurosci. 1986;6:3505–3517. doi: 10.1523/JNEUROSCI.06-12-03505.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeVries S H, Baylor D A. Proc Natl Acad Sci USA. 1995;92:10658–10662. doi: 10.1073/pnas.92.23.10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soucy E, Wang Y, Nirenberg S, Nathans J, Meister M. Neuron. 1998;21:481–493. doi: 10.1016/s0896-6273(00)80560-7. [DOI] [PubMed] [Google Scholar]
- 29.Vissavajjhala P, Janssen W G M, Hu Y, Gazzaley A H, Moran T, Hof P R, Morrison J H. Exp Neurol. 1996;142:296–312. doi: 10.1006/exnr.1996.0199. [DOI] [PubMed] [Google Scholar]
- 30.Petralia R S, Wang Y-X, Mayat E, Wenthold R J. J Comp Neurol. 1997;385:456–476. doi: 10.1002/(sici)1096-9861(19970901)385:3<456::aid-cne9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 31.Brandstätter J H, Koulen P, Kuhn R, van der Putten H, Wässle H. J Neurosci. 1996;16:4749–4756. doi: 10.1523/JNEUROSCI.16-15-04749.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koulen P, Kuhn R, Wässle H, Brandstätter J H. J Neurosci. 1997;17:2200–2211. doi: 10.1523/JNEUROSCI.17-06-02200.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cai W, Pourcho R G. J Comp Neurol. 1999;407:427–437. doi: 10.1002/(sici)1096-9861(19990510)407:3<427::aid-cne10>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 34.Koulen P, Kuhn R, Wässle H, Brandstätter J H. Proc Natl Acad Sci USA. 1999;96:9909–9914. doi: 10.1073/pnas.96.17.9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morigiwa K, Vardi N. J Comp Neurol. 1999;405:173–184. doi: 10.1002/(sici)1096-9861(19990308)405:2<173::aid-cne3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 36.Raviola E, Gilula N B. Proc Natl Acad Sci USA. 1973;70:1677–1681. doi: 10.1073/pnas.70.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kolb H. J Neurocytol. 1977;6:131–153. doi: 10.1007/BF01261502. [DOI] [PubMed] [Google Scholar]
- 38.Nelson R. J Comp Neurol. 1977;172:109–136. doi: 10.1002/cne.901720106. [DOI] [PubMed] [Google Scholar]
- 39.Schneeweis D M, Schnapf J L. Science. 1995;268:1053–1056. doi: 10.1126/science.7754386. [DOI] [PubMed] [Google Scholar]
- 40.Hollmann M, Heinemann S. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- 41.West R W. Vision Res. 1978;18:129–136. doi: 10.1016/0042-6989(78)90177-3. [DOI] [PubMed] [Google Scholar]
- 42.Sterling P. Neuron. 1998;21:643–644. doi: 10.1016/s0896-6273(00)80574-7. [DOI] [PubMed] [Google Scholar]