Abstract
Cannabinoids, including the endogenous ligand arachidonyl ethanolamide (anandamide), elicit not only neurobehavioral but also cardiovascular effects. Two cannabinoid receptors, CB1 and CB2, have been cloned, and studies with the selective CB1 receptor antagonist SR141716A have implicated peripherally located CB1 receptors in the hypotensive action of cannabinoids. In rat mesenteric arteries, anandamide-induced vasodilation is inhibited by SR141716A, but other potent CB1 receptor agonists, such as HU-210, do not cause vasodilation, which implicates an as-yet-unidentified receptor in this effect. Here we show that “abnormal cannabidiol” (Abn-cbd) is a neurobehaviorally inactive cannabinoid that does not bind to CB1 receptors, yet causes SR141716A-sensitive hypotension and mesenteric vasodilation in wild-type mice and in mice lacking CB1 receptors or both CB1 and CB2 receptors. Hypotension by Abn-cbd is also inhibited by cannabidiol (20 μg/g), which does not influence anandamide- or HU-210-induced hypotension. In the rat mesenteric arterial bed, Abn-cbd-induced vasodilation is unaffected by blockade of endothelial NO synthase, cyclooxygenase, or capsaicin receptors, but it is abolished by endothelial denudation. Mesenteric vasodilation by Abn-cbd, but not by acetylcholine, sodium nitroprusside, or capsaicine, is blocked by SR141716A (1 μM) or by cannabidiol (10 μM). Abn-cbd-induced vasodilation is also blocked in the presence of charybdotoxin (100 nM) plus apamin (100 nM), a combination of K+-channel toxins reported to block the release of an endothelium-derived hyperpolarizing factor (EDHF). These findings suggest that Abn-cbd and cannabidiol are a selective agonist and antagonist, respectively, of an as-yet-unidentified endothelial receptor for anandamide, activation of which elicits NO-independent mesenteric vasodilation, possibly by means of the release of EDHF.
Cannabinoids, the biologically active constituents of marijuana, have been used for millennia for their psychoactive properties. The marijuana plant contains more than 60 distinct chemical substances, of which Δ9-tetrahydrocannabinol (THC) is the main psychoactive ingredient (1). In 1992, the first endogenous cannabinoid was isolated from porcine brain and identified as arachidonyl ethanolamide (anandamide) (2). In addition to having their well known neurobehavioral effects, THC and anandamide influence a number of other physiological functions, including cardiovascular variables (3). In experimental animals, THC elicits prolonged hypotension and bradycardia (4), and similar effects have been described for anandamide (5). Although early studies implicated centrally mediated sympatho-inhibition in these effects (4), cannabinoids can also induce hypotension by presynaptic inhibition of norepinephrine release from peripheral sympathetic nerve terminals (6–9) or by direct vasodilation (10, 11). To date, two cannabinoid receptors have been cloned, the CB1 receptor expressed primarily in the brain (12) but also in some peripheral tissues (6, 13), and CB2 receptors expressed by cells of the immune system (14). Studies with the selective CB1 receptor antagonist SR141716A implicated the CB1 receptor subtype in cannabinoid-induced hypotension and bradycardia (5, 10), a conclusion recently confirmed by the use of mice deficient in CB1 receptors (15). However, we recently reported (16) that in the rat isolated mesenteric arterial bed anandamide elicits prolonged vasodilation partially inhibited by SR141716A, but THC and synthetic agonists highly potent at both CB1 and CB2 receptors, such as HU-210 (17) or WIN 55212–2 (18), do not have a vasodilator effect. These findings implicate as-yet-unidentified receptor(s) in anandamide-induced mesenteric vasodilation.
In the present study we demonstrate that anandamide-induced mesenteric vasodilation persists in mice deficient in CB1 receptors or in both CB1 and CB2 receptors. Two cannabinoid analogs devoid of neurobehavioral activity are identified that act as a selective agonist or antagonist, respectively, at an endothelial site responsible for the SR141716A-sensitive, endothelium-dependent component of anandamide-induced mesenteric vasodilation. Additional evidence suggests that the vasodilation triggered from this site is nitric oxide (NO)-independent and may be mediated by the release of an endothelium-derived hyperpolarizing factor (EDHF).
Materials and Methods
Animals.
CB1 receptor knockout (CB1−/−) mice and their homozygous controls (CB1+/+ mice) were developed in C57BL6J mice by replacing most of the CB1 receptor coding sequence with a nonreceptor sequence through homologous recombination in MPI2 embryonic stem cells, as detailed elsewhere (19).‖ CB1−/−/CB2−/− double knockout mice were obtained with the expected mendelian frequency by mating mice heterozygous for both receptors (CB1+/−/CB2+/−). The heterozygote mice, in turn, were obtained by mating CB1−/− mice with CB2−/− mice. The CB2−/− mice were obtained by disrupting the CB2 gene by using homologous recombination in the embryonic stem cell line 129 (N.E.B., K. L. McCoy, C. C. Felder, M. Glass, E.M., T.I.B., A.M.Z., and A.Z., unpublished results). The CB1−/−/CB2−/− mice are healthy, are of size and weight similar to their wild-type littermates, and have no gross defects. They are fertile, and care for their offspring. Brain and spleen tissues from the CB1−/−/CB2−/− mice do not express CB1 or CB2 mRNA as revealed by in situ hybridization histochemistry, and do not contain specific binding sites for the potent CB1/CB2 agonist [3H]CP-55,940 (N.E.B., E.M., T.I.B., A.M.Z., and A.Z., unpublished observations).
Adult ICR mice (25–30 g) were from Harlan (Indianapolis, IN). Both male and female animals were used for these studies. Animals were housed four in a cage with standard mouse chow and water ad libitum and were maintained at 24–26°C under a 12:12 light:dark cycle.
Cardiovascular Measurements.
The effect of cannabinoids on blood pressure was tested in anesthetized rather than conscious mice, as anesthesia was found to potentiate the CB1 receptor-mediated hypotensive effect of anandamide (20). Male and female mice (25–30 g) were anesthetized with sodium pentobarbital, 60 mg/kg i.p., and PE10 cannulae were inserted into the carotid artery and jugular vein for continuous monitoring of blood pressure and heart rate and for injecting drugs, respectively. Drugs were injected as bolus i.v. doses in volumes ≤50 μl. Injection of vehicle caused no significant change in blood pressure.
For measuring drug effects on mesenteric vascular tone in mice, sodium pentobarbital-anesthetized mice were laparotomized and a PE50 cannula was inserted distally into the abdominal aorta. Both renal and both femoral arteries were ligated, the heart was removed, and the mesenteric area including the liver was perfused with oxygenated Krebs buffer at 36°C, using a peristaltic pump and a constant flow rate of 0.7 ml/min. Perfusion pressure monitored near the inflow cannula was 25–30 mmHg (1 mmHg = 133 Pa), and was increased to 60–70 mmHg by the inclusion of 15 μM phenylephrine in the medium. Vasodilation was expressed as percent relaxation of established tone, 100% being equal to the difference in perfusion pressure in the absence and presence of phenylephrine.
The methods used to analyze drug effects on vascular tone in the rat isolated mesenteric arterial bed and to achieve endothelial denudation by brief perfusion with distilled water have been described in detail elsewhere (16). Because of the long lasting vasodilator effect of anandamide, (R)-methanandamide, and “abnormal cannabidiol” (Abn-cbd), each preparation was tested with a single dose of the agonist, either in the absence or in the presence of an antagonist.
Neurobehavioral Tests.
Core body temperature, tail flick latency, locomotor activity, and ring immobility tests were conducted in unanesthetized mice as detailed elsewhere (21). Drug effects in the above four tests were expressed, respectively, as Δ°C, % MPE (percent of maximal possible effect, which is the difference of the basal tail flick latency of 2–3 s and an arbitrary cutoff at 10 s), percent change of photocell beam interruptions over a 10-min period, and percent of time spent motionless on a ring over a 5-min period. Abn-cbd or vehicle was injected i.v. into a tail vein, and measurements were done at 5 min (spontaneous motility), 20 min (tail flick latency), 60 min (core temperature), or 90 min (ring immobility) after drug injection (21).
Radioligand Binding.
[3H]CP-55,940 binding to P2 membranes from rat brain was conducted as described elsewhere (22). In saturation experiments (n = 5), the Bmax (1.2 ± 0.2 pmol/mg protein) and Kd values (809 ± 21 pM) were similar to published values (22).
Drugs.
HU-210 is 3-(1,1-dimethylheptyl)-(−)-11-OH-Δ9-THC, SR141716A is N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide⋅hydrochloride. The structures of the other cannabinoid ligands mentioned in the text have been described elsewhere (10). Abn-cbd, (−)-4-(3-3,4-trans-p-menthadien-1,8)-yl-olivetol (see Fig. 3D), results from the transposition of the phenolic hydroxyl group and the pentyl side chain of cannabidiol. It was synthesized as described (23), and its purity was established by nuclear magnetic resonance spectroscopy, gas chromatography, and elemental analysis. Cannabinoids were dissolved in Emulphor/ethanol/saline, 1:1:18.
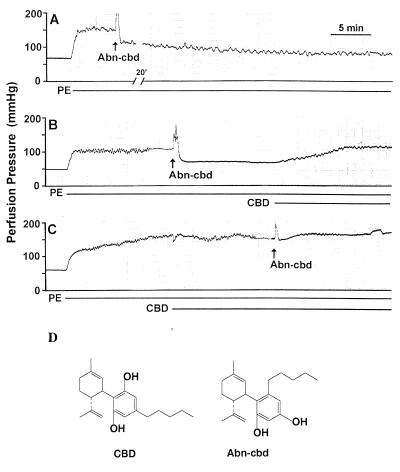
Figure 3.
Vasodilator action of Abn-cbd and its antagonism by cannabidiol (CBD) in the rat isolated mesenteric arterial bed. Each panel represents a separate preparation, illustrating the long lasting vasodilation by 4 mg of Abn-cbd (A), and its reversal (B) or prevention (C) by 10 μM CBD. Phenylephrine (PE, 15 μM) used to precontract the preparation and CBD were included in the perfusion buffer, as indicated by the horizontal lines, and the bolus injection of 4 mg of Abn-cbd is indicated by the arrows. These experiments were replicated in 4–6 additional preparations with similar results. (D) Structures of CBD and Abn-cbd.
Statistical Analyses.
The time-dependent effects of drugs on blood pressure were analyzed by ANOVA followed by Bonferroni’s test. Agonist-induced mesenteric vasodilator (percent relaxation) responses in the absence or presence of an antagonist were compared with Student’s unpaired t test. Differences with a P value of <0.05 were considered statistically significant.
Results
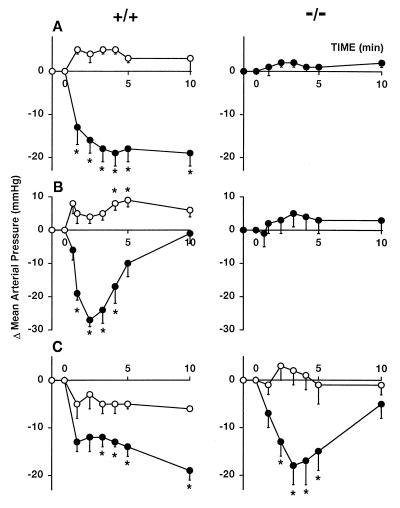
Fig. 1 summarizes the changes in mean arterial pressure in pentobarbital-anesthetized CB1 receptor knockout mice (CB1−/−) and their control littermates (CB1+/+) in response to the potent synthetic cannabinoid HU-210 (A) and anandamide (B). The doses used were submaximal for hypotension, as deduced from dose–response studies in wild-type ICR mice (not shown). In CB1+/+ mice, both compounds elicited prolonged hypotension, blocked by pretreatment with SR141716A, whereas these effects were completely absent in CB1−/− mice. Anesthetized CB1−/− and CB1+/+ mice had similar basal blood pressure (74 ± 2 vs. 75 ± 2 mmHg, n = 41 vs. 45), which is compatible with earlier findings of a lack of effect on this parameter by SR141716A (10). Both of these findings suggest that CB1 receptors involved in the control of blood pressure are not tonically active.
Figure 1.
Effects of HU-210 (10 ng/g; A), anandamide (4 μg/g; B), and Abn-cbd (20 μg/g; C) in the absence (●) and presence of 3 μg/g SR141716A (○) on mean arterial blood pressure in pentobarbital-anesthetized CB1+/+ (Left) and CB1−/− (Right) mice. Mice were injected with a bolus i.v. dose of the indicated agonist at 0 min, 10 min after the injection of vehicle or SR141716A. Points and bars represent means ± SE from 4–7 animals. * indicate significant difference (P < 0.05) from corresponding baseline (0) values.
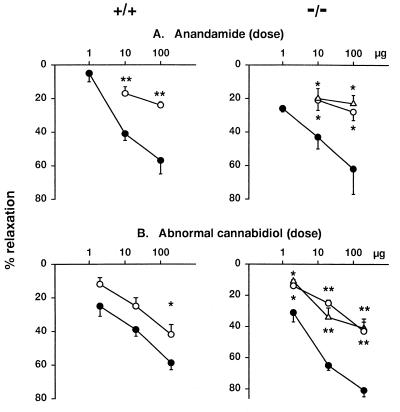
Because recent findings suggest that anandamide-induced mesenteric vasodilation is mediated by receptors other than CB1 cannabinoid receptors (16), we tested whether such an effect is present in CB1−/− mice. Intra-arterial injection of anandamide into the mesenteric vasculature caused dose-dependent, prolonged but reversible vasodilation, which was somewhat greater in CB1−/− than in CB1+/+ mice and was inhibited in the presence of 1 or 5 μM SR141716A in the perfusion medium (Fig. 2A). At the 5 μM concentration, SR141716A did not inhibit the vasodilator response to 3 μg of sodium nitroprusside (54% ± 4%, vs. 49% ± 3%, n = 10). Anandamide in tissues is rapidly degraded into arachidonic acid and ethanolamine by an amidohydrolase (24). However, its mesenteric vasodilator effect could not be caused by a degradation product, because the metabolically stable (R)-methanandamide (25) also caused vasodilation (44% ± 5% at 10 μg, n = 5, and 50% ± 5% at 100 μg, n = 4), which was inhibited by 5 μM SR141716A (10% ± 5% at 10 μg, n = 4, P < 0.005, and 23% ± 3% at 100 μg, n = 4, P < 0.005). The finding that (R)-methanandamide caused similar mesenteric vasodilation in CB1−/−/CB2−/− double-knockout mice (10 μg: 34% ± 4%, n = 3; 100 μg: 48% ± 2%, n = 3) excluded the role of CB2 receptors in this effect. These findings lend further support to the existence of one or more as-yet-undefined receptors in the mesenteric vasculature, for which anandamide and (R)-methanandamide are agonists and SR141716A is an antagonist. Although the inhibitory potency of SR141716A appears somewhat lower than its potency for CB1 receptors (26), significant inhibition was observed at a concentration of 1 μM, which does not cause the direct inhibition of Ca2+ or K+ channels observed at higher concentrations of SR141716A (27).
Figure 2.
Mesenteric vasodilator effect of anandamide (A) and abnormal cannabidiol (B) in the absence (●) or presence of SR141716A (1 μM ▵ or 5 μM ○) in CB1+/+ (Left) and CB1−/− mice (Right). Agonists were injected as an intraarterial bolus, whereas SR141716A was included in the perfusion buffer. Points and vertical bars represent means ± SE from 3–6 experiments. Each preparation was used for one agonist injection only. *, P < 0.05; **, P < 0.005 from corresponding value in the absence of SR141716A.
Abn-cbd (see Fig. 3D) is a synthetic cannabinoid analog that was reported to lack cannabinoid-like behavioral effects in two paradigms but to cause profound hypotension in dogs (28). Because these findings suggest that Abn-cbd may be a selective agonist at the putative vascular anandamide receptor, we synthesized Abn-cbd and tested its neurobehavioral and cardiovascular effects and its CB1 receptor binding activity. Abn-cbd given i.v. to ICR mice in doses of 15–60 μg/g was inactive in a tetrad of tests considered diagnostic for cannabinoid action (22), whereas 3 μg/g THC, which causes the same degree of hypotension in CB1+/+ mice as 20 μg/g Abn-cbd, was strongly active (Table 1). In ligand binding assays using rat brain plasma membranes, Abn-cbd (0.03 to 100 μM) did not displace [3H]CP-55,940, a potent cannabinoid agonist (displacement at 100 μM: 6% ± 9% of specific binding, n = 4). However, Abn-cbd elicited dose-dependent hypotension (5–30 μg/g i.v., ED50: 7.6 ± 0.6 μg/g) without affecting heart rate in anesthetized CB1+/+ and CB1−/− mice, and the hypotension was blocked by pretreatment with 3 μg/g SR141716A in both groups (Fig. 1C). Abn-cbd (20 μg/g) also elicited hypotension in CB1−/−/CB2−/− mice (−24 ± 5 mmHg, n = 4), which was similar in magnitude and duration to that seen in CB1−/− mice. In the buffer-perfused mesenteric vascular bed of CB1−/− mice, Abn-cbd elicited pronounced and long lasting vasodilation, which was significantly inhibited by 1–5 μM SR141716A (Fig. 2B). In mesenteric preparations from CB1−/−/CB2−/− mice, Abn-cbd (200 μg) caused vasodilation (75% ± 4%, n = 4) similar to that in preparations from CB1−/− mice. These findings indicate that Abn-cbd acts at a vascular site other than CB1 or CB2 receptors.
Table 1.
Neurobehavioral effects of Abn-CBD and THC in the mouse model of cannabinoid activity
| Treatment | Locomotor activity, % inhibition | Antinociception, % MPE | Core temp, Δ°C | Ring immobility, % |
|---|---|---|---|---|
| Vehicle | 0 ± 0 | 6 ± 3 | 0.5 ± 0.1 | 0 ± 0 |
| Abn-cbd (60 μg/g) | 6 ± 16 | 13 ± 3 | −0.5 ± 0.9 | 0 ± 0 |
| THC (3 μg/g) | 79 ± 6** | 92 ± 8** | −4.5 ± 0.5** | 34 ± 5* |
Responses were expressed as described in Materials and Methods, MPE, maximal possible effect. Means ± SE from 6–12 independent experiments are shown. Significant difference from corresponding responses in vehicle-treated mice: *,P < 0.05; **, P < 0.005.
The vasodilator response to Abn-cbd was analyzed in more detail by using the rat isolated mesenteric arterial bed preparation. Vasodilator mechanisms have been much more widely documented in this preparation than in the mouse mesentery and, in our hands, reliable endothelial denudation could be achieved only in the rat preparation. Abn-cbd caused dose-dependent (ED50: 2.46 ± 0.15 mg), prolonged vasodilation (see Fig. 3A). Vasodilation in response to the maximally effective dose of Abn-cbd was strongly inhibited in the presence of 1 μM SR141716A and was absent when tested after endothelial denudation (see Fig. 5). This observation is in agreement with earlier findings that the SR141716A-sensitive component of anandamide-induced mesenteric vasodilation is endothelium dependent (16). However, anandamide can also relax mesenteric vascular smooth muscle by a noncannabinoid mechanism (16), which Abn-cbd cannot.
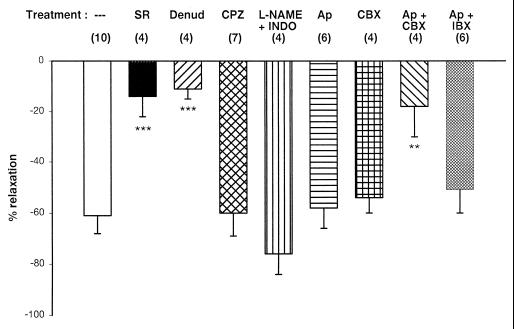
Figure 5.
Analysis of the mechanism of the vasodilator action of Abn-cbd in the rat isolated mesenteric arterial bed preparation. Columns and bars represent the means ± SE for the vasodilator response to 4 mg of Abn-cbd under the following conditions: control (open column); endothelium-denuded preparations (Denud); endothelium-intact preparations perfused with 1 μM SR141716A (SR); 5 μM capsazepine (CPZ); 100 μM NG-nitro-l-arginine methyl ester plus 10 μM indomethacin (L-NAME + INDO); 100 nM apamin (Ap); 100 nM charybdotoxin (CBX); 100 nM apamin plus 100 nM charybdotoxin (Ap + CBX); or 100 nM apamin plus 50 nM iberiotoxin (Ap + IBX). Numbers of preparations tested are in parentheses. For explanations, see text. **, P < 0.01; ***, P < 0.005.
To begin to examine the structural requirements for the vasodilator activity of Abn-cbd, we synthesized O-1602, an analog of Abn-cbd in which the pentyl side chain was shortened to a methyl group. Similar to Abn-cbd, O-1602 did not bind to CB1 receptors in ligand binding assays. In the rat isolated mesenteric vascular bed, O-1602 was a full agonist in causing dose-dependent vasodilation, and it was ≈80 times more potent than Abn-cbd (ED50: 30.5 ± 1.5 μg). Similar to Abn-cbd, the vasodilation induced by O-1602 (54% ± 2% at 100 μg, n = 4) was markedly reduced in the presence of 1 μM SR141716A (15% ± 3%, n = 3, P < 0.005) or after endothelial denudation (18% ± 2%, n = 3, P < 0.005).
Cannabidiol, the parent compound of Abn-cbd (see Fig. 3D), is a naturally occurring constituent of cannabis devoid of both neurobehavioral (29) and hypotensive (28) activity. We tested cannabidiol for potential antagonism of the mesenteric vasodilator effect of Abn-cbd. The inclusion of 10 μM cannabidiol in the perfusion medium did not significantly alter perfusion pressure, but prevented the dilator response to the subsequent intraarterial injection of 4 mg of Abn-cbd (Fig. 3C), or caused its rapid reversal when administered during the plateau phase of the dilator response (Fig. 3B). The same concentration of cannabidiol also significantly attenuated the vasodilator response to anandamide (Fig. 4), but not the responses to 10 μg of acetylcholine (88% ± 9% vs. 78% ± 5% in controls, n = 4), 10 μg of bradykinin (77% ± 6% vs. 70% ± 4%, n = 4) or 10 μg of sodium nitroprusside (76% ± 2% vs. 78% ± 7%, n = 5). In anesthetized mice, the i.v. administration of 20 μg/g cannabidiol did not influence the CB1 receptor-mediated hypotensive response to submaximal hypotensive doses of anandamide or HU-210, but significantly inhibited Abn-cbd-induced hypotension (Δ mean arterial pressure: −20 ± 2 vs. −8 ± 2 mmHg, n = 6, P < 0.01). These findings suggest that cannabidiol may be a selective antagonist of the endothelial anandamide receptor.
Figure 4.
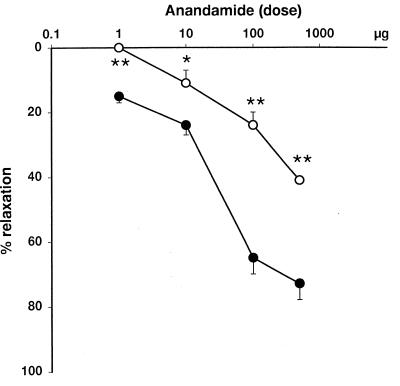
Anandamide-induced vasodilation (●) is inhibited in the presence 10 μM cannabidiol (○) in rat mesenteric arteries. Points and bars represent means ± SE from 4 or 5 experiments. Asterisks indicate statistically significant difference from corresponding control values: (*, P < 0.05; **, P < 0.005).
We also analyzed the respective role of endothelial nitric oxide (NO), cyclooxygenase, and potassium channels in Abn-cbd-induced mesenteric vasodilation. The vasodilator response to 4 mg of Abn-cbd remained unchanged in the presence of 100 μM NG-nitro-l-arginine methyl ester (l-NAME) and 10 μM indomethacin in the perfusion buffer, which indicates that endothelial NO and cyclooxygenase products such as prostacyclin do not contribute to the response (Fig. 5). The individual presence of 100 nM apamin or 100 nM charybdotoxin, inhibitors of different Ca2+-activated K+ channels, also did not inhibit Abn-cbd-induced mesenteric vasodilation (Fig. 5), although in the presence of apamin the effect was shorter lasting than in its absence. However, the combined presence of these two toxins strongly inhibited the response to Abn-cbd (Fig. 5), similar to their reported ability to block anandamide-induced mesenteric vasodilation (30), whereas the combination of apamin plus iberiotoxin was ineffective (Fig. 5).
Because of structural similarities between anandamide and some synthetic agonists of capsaicine-sensitive vanilloid receptors (31), we examined the effect of the vanilloid receptor antagonist capsazepine on Abn-cbd-induced vasodilation in the endothelium-intact rat mesenteric arterial preparation. In the presence of 5 μM capsazepine (Kd at vanilloid VR1 receptors: 285 nM), the bolus injection of 4 mg of Abn-cbd elicited the same long lasting vasodilation as in its absence (Fig. 5). As illustrated in Fig. 6, the same concentration of capsazepine caused the expected significant right-shift of the dose–response curve for capsaicin-induced vasodilation, which confirms that VR1 vanilloid receptors were indeed inhibited. As also shown in Fig. 6, the effect of capsaicine remained unchanged in the presence of 1 μM SR141716A, which indicates that inhibition of the effect of Abn-cbd by 1 μM SR141716A (see Fig. 5) cannot be attributed to nonspecific blockade of VR1 receptors.
Figure 6.
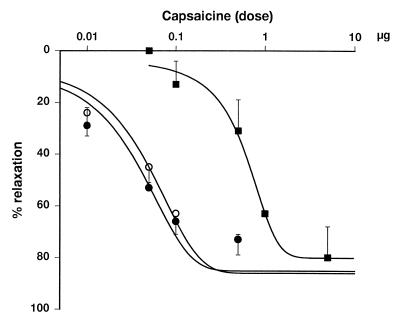
Capsaicine-induced mesenteric vasodilation is competitively inhibited by capsazepine, but not by SR141716A. Vasodilator responses to intraarterial bolus injections of capsaicine were tested in the rat isolated mesenteric vascular bed under control conditions (●), in the presence of 5 μM capsazepine (■) or in the presence of 1 μM SR141716A (○). Points and vertical bars represent means ± SE from 3–5 experiments.
Discussion
On the basis of classical pharmacological criteria, such as the rank order of agonist potencies and inhibition by a selective antagonist, the hypotensive effect of cannabinoids, including that of the endogenous ligand anandamide, has been attributed to the activation of the CB1 subtype of cannabinoid receptors (10). A similar pharmacological approach led to the unexpected conclusion that CB1 receptors are not involved in the mesenteric vasodilator effect of anandamide (16), even though the effect could be inhibited by the CB1 receptor antagonist SR141716A (16, 30, 32). Through the use of mice deficient in CB1 receptors, the present studies provide definitive evidence for both hypotheses: the hypotensive effect of anandamide is lost in CB1−/− mice, whereas its SR141716A-sensitive mesenteric vasodilator effect is maintained or even increased compared with responses in CB1+/+ mice. Thus, anandamide is able to elicit cardiovascular depressor effects by both CB1 receptor-dependent and -independent mechanisms. These findings also indicate that the non-CB1 receptor-mediated mesenteric vasodilator effect of anandamide does not significantly contribute to its hypotensive action in vivo, at least in the case of exogenous anandamide. Anandamide is known to have a non-CB1 receptor-mediated pressor effect (refs. 5 and 10; see also Fig. 1B), which may counteract decreases in vascular resistance limited to certain vascular beds. Also, the compensatory increase in sympathetic tone normally triggered by vasodilation would be limited by the simultaneous action of anandamide at presynaptic CB1 receptors in wild-type animals (6–9) but not in CB1−/− mice.
Our previous findings highlighted an additional complexity in the mesenteric vasodilator action of anandamide, which is, to a large degree, endothelium-independent, and only a relatively minor endothelium-dependent component is sensitive to inhibition by low concentrations of SR141716A (16). The evidence presented here indicates that Abn-cbd may be a selective agonist at this latter site. Similar to anandamide, Abn-cbd elicits SR141716A-sensitive mesenteric vasodilation, but it is neurobehaviorally inactive and does not interact with CB1 receptors even at very high concentrations. In further contrast to the effect of anandamide, mesenteric vasodilation by Abn-cbd is abolished by endothelial denudation. The persistence of the SR141716A-sensitive mesenteric vasodilator effect of Abn-cbd in CB1−/− mice rules out the involvement of CB1 receptors and implicates a non-CB1 site of action of SR141716A.
A recent report has demonstrated that micromolar concentrations of anandamide bind to vanilloid receptors, whereas much lower concentrations trigger the release of calcitonin gene-related peptide in certain isolated blood vessels, and that inhibition of this process antagonizes anandamide-induced vasodilation (33). However, the endothelium-dependent vasodilator effect of Abn-cbd and anandamide cannot be due to such a mechanism. The vanilloid receptor antagonist capsazepine did not influence the effect of Abn-cbd at a concentration at which it significantly inhibited vasodilation by capsaicin and, conversely, SR141716A did not influence the response to capsaicin at a concentration (1 μM) at which it significantly inhibited the effect of Abn-cbd. Because SR141716A does not inhibit vanilloid receptors (Fig. 6), a role of such receptors is likely limited to the endothelium-independent, SR141716A-resistant component of the effect of anandamide (16). Indeed, in preliminary experiments we found that in endothelium-denuded rat mesenteric arteries, in which Abn-cbd does not cause vasodilation (Fig. 5), the SR141716A-resistant vasodilator response to anandamide was inhibited by 5 μM capsazepine. Thus, the mechanism of the vasodilator effect of anandamide is complex: in addition to the demonstrated involvement of CB1 receptors in some blood vessels (34), it has an SR141716A-sensitive component mediated by an as-yet-undefined endothelial receptor, and an endothelium-independent, SR141176A-resistant component likely mediated by vanilloid receptors.
Nonspecific blockade of cation channels is also unlikely to account for the inhibitory effect of SR141716A. SR141716A, at a concentration of 10 μM but not at 1 μM, has been reported to inhibit vasorelaxation caused by direct activation of K+ channels (27) and to nonselectively inhibit agonist-induced Ca2+ mobilization in endothelial cells at 5 μM but not at 1 μM (35). In the present experiments, 1 μM SR141716A nearly completely blocked the vasodilator effect of a maximally effective dose of Abn-cbd.
Unlike SR141716A, an inhibitor of both CB1 receptors and the endothelial site of action of Abn-cbd and anandamide, cannabidiol may be a selective inhibitor of the latter. Cannabidiol, a natural constituent of the marijuana plant, is similar to Abn-cbd in that it is neurobehaviorally inactive (29, 36). Unlike Abn-cbd, cannabidiol does not elicit mesenteric vasodilation, but it is able to prevent or reverse the effect of Abn-cbd (Fig. 3) and also to partially inhibit the vasodilator effect of anandamide. Cannabidiol also inhibits the in vivo hypotensive effect of Abn-cbd, but not the CB1 receptor-mediated hypotensive effects of anandamide or HU-210. This spectrum of activity suggests that cannabidiol is a selective antagonist of the endothelial receptor for Abn-cbd and anandamide described here, and also supports the idea that the in vivo and in vitro effects of Abn-cbd are mediated by similar mechanisms. The apparent selectivity of cannabidiol as an antagonist and the indications of a structure–activity relationship for Abn-cbd and its analog O-1602 strongly suggest that the site of action of these compounds is a receptor.
The unchanged vasodilator response to Abn-cbd in the presence of inhibitors of endothelial NO synthase and cyclooxygenase discounts the role of NO and cyclooxygenase products in this effect. However, it is possible that a compensatory increase in NO-independent pathways triggered by the inhibitors may mask an NO-mediated component present in untreated preparations, by analogy to the reported up-regulation of EDHF effects in the presence of inhibitors of NO synthase (37). Blockade of Abn-cbd-induced vasodilation by a combination of apamin plus charybdotoxin but not apamin plus iberiotoxin is reminiscent of blockade of the effects of EDHF by the same combination of toxins (30, 38, 39), where the observed inhibition was attributed to blockade of the release of EDHF from the endothelium (39). Therefore, these findings suggest that Abn-cbd causes NO-independent vasodilation in rat mesenteric arteries by triggering the release of an EDHF, such as K+ itself (39) or an epoxygenase product (40). Vascular endothelial cells contain Ca2+-activated K+ channels, which are opened by a rise in intracellular calcium (41), and anandamide has been shown to induce calcium transients in vascular endothelial cells by the activation of an SR141716A-sensitive mechanism (35, 42). It has been proposed that EDHF itself may be an endocannabinoid acting on CB1-like receptors in vascular smooth muscle, based on the ability of SR141716A to inhibit EDHF-induced mesenteric vasodilation (30). The present findings are not compatible with this proposal, because Abn-cbd loses its vasodilator effect after endothelial denudation, and the residual dilator response to anandamide is no longer sensitive to inhibition by SR141716A (16). However, endocannabinoids have been identified in vascular endothelium (43, 44), and it is possible that in vessels in which SR141716A was found to inhibit the effect of EDHF, acetylcholine may cause the luminal release of an endocannabinoid, which then acts on an endothelial receptor to release EDHF into the myoendothelial junction.
Regardless of the cellular mechanisms involved, the availability of a selective, nonpsychoactive agonist and antagonist for cannabinoid-induced vasodilation may have therapeutic implications in vascular diseases, and such compounds may be used as tools for identifying their molecular site of action.
Acknowledgments
We thank Dr. Francis Barth for providing SR141716A and Lei Wang for technical assistance. This work was supported by grants from the National Institutes of Health. Z.J. and J.A.W. were supported by fellowships from Sanofi Research, Inc., and the Deutsche Forschungsgemeinschaft, respectively.
Abbreviations
- THC
Δ9-tetrahydrocannabinol
- EDHF
endothelium-derived hyperpolarizing factor
- Abn-cbd
abnormal cannabidiol
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Inquiries about the knockout mice only should be directed to A.Z. at zimmer@codon.nih.gov.
References
- 1.Dewey W L. Pharmacol Rev. 1986;38:151–178. [PubMed] [Google Scholar]
- 2.Devane W A, Hanus L, Breuer A, Pertwee R G, Stevenson L A, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 3.Wagner J A, Varga K, Kunos G. J Mol Med. 1998;76:824–836. doi: 10.1007/s001090050287. [DOI] [PubMed] [Google Scholar]
- 4.Vollmer R R, Cavero I, Ertel R J, Solomon T A, Buckley J P. J Pharm Pharmacol. 1974;26:186–192. doi: 10.1111/j.2042-7158.1974.tb09252.x. [DOI] [PubMed] [Google Scholar]
- 5.Varga K, Lake K, Martin B R, Kunos G. Eur J Pharmacol. 1995;278:279–283. doi: 10.1016/0014-2999(95)00181-j. [DOI] [PubMed] [Google Scholar]
- 6.Varga K, Lake K D, Huangfu D, Guyenet P G, Kunos G. Hypertension. 1996;28:682–686. doi: 10.1161/01.hyp.28.4.682. [DOI] [PubMed] [Google Scholar]
- 7.Ishac E J N, Jiang L, Lake K D, Varga K, Abood M, Kunos G. Br J Pharmacol. 1996;118:2023–2028. doi: 10.1111/j.1476-5381.1996.tb15639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malinowska B, Godlewski G, Bucher B, Schlicker E. Naunyn-Schmiedebergs Arch Pharmacol. 1997;356:197–202. doi: 10.1007/pl00005041. [DOI] [PubMed] [Google Scholar]
- 9.Niederhoffer N, Szabo B. Br J Pharmacol. 1999;126:457–466. doi: 10.1038/sj.bjp.0702337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lake K D, Compton D R, Varga K, Martin B R, Kunos G. J Pharmacol Exp Ther. 1997;281:1030–1037. [PubMed] [Google Scholar]
- 11.Vidrio H, Sanchez-Salvatori M A, Medina M. J Cardiovasc Pharmacol. 1996;28:332–336. doi: 10.1097/00005344-199608000-00022. [DOI] [PubMed] [Google Scholar]
- 12.Matsuda L A, Lolait S J, Brownstein M J, Young A C, Bonner T I. Nature (London) 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 13.Shire D, Carillon C, Kaghad M, Calandra B, Rinaldi-Carmona M, Le Fur G, Ferrara P. J Biol Chem. 1995;270:3726–3731. doi: 10.1074/jbc.270.8.3726. [DOI] [PubMed] [Google Scholar]
- 14.Munro S, Thomas K L, Abu-Shaar M. Nature (London) 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 15.Ledent C, Valverde O, Cossu G, Petitet F, Aubert J-F, Beslot F, Böhme G A, Imperato A, Pedrazzini T, Roques B P, et al. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- 16.Wagner J A, Varga K, Járai Z, Kunos G. Hypertension. 1999;33(Part II):429–434. doi: 10.1161/01.hyp.33.1.429. [DOI] [PubMed] [Google Scholar]
- 17.Howlett A C, Champion T M, Wilken G H, Mechoulam R. Neuropharmacology. 1990;29:161–165. doi: 10.1016/0028-3908(90)90056-w. [DOI] [PubMed] [Google Scholar]
- 18.Felder C C, Joyce K E, Briley E M, Mansouri J, Mackie K, Blond O, Lai Y, Ma A L, Mitchell R L. Mol Pharmacol. 1995;48:443–450. [PubMed] [Google Scholar]
- 19.Zimmer A, Zimmer A M, Hohmann A G, Herkenham M, Bonner T I. Proc Natl Acad Sci USA. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lake K D, Martin B R, Kunos G, Varga K. Hypertension. 1997;29:1204–1210. doi: 10.1161/01.hyp.29.5.1204. [DOI] [PubMed] [Google Scholar]
- 21.Smith P B, Compton D R, Welch S P, Razdan R K, Mechoulam R, Martin B R. J Pharmacol Exp Ther. 1994;270:219–227. [PubMed] [Google Scholar]
- 22.Compton D R, Rice K C, de Costa B R, Razdan R K, Melvin L S, Johnson M R, Martin B R. J Pharmacol Exp Ther. 1993;265:218–226. [PubMed] [Google Scholar]
- 23.Razdan R K, Dalzell H C, Handrick G R. J Am Chem Soc. 1974;96:5860–5865. doi: 10.1021/ja00825a026. [DOI] [PubMed] [Google Scholar]
- 24.Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz J-C, Piomelli D. Nature (London) 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- 25.Abadji V, Lin S, Taha G, Griffin G, Stevenson L A, Pertwee R G, Makriyannis A. J Med Chem. 1994;37:1889–1893. doi: 10.1021/jm00038a020. [DOI] [PubMed] [Google Scholar]
- 26.Rinaldi-Carmona M, Barth F, Heaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Neliat G, Caput D, et al. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- 27.White R, Hiley C R. Br J Pharmacol. 1998;125:689–696. doi: 10.1038/sj.bjp.0702127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adams M D, Earnhardt J T, Martin B R, Harris L S, Dewey W L, Razdan R K. Experientia. 1977;33:1204–1205. doi: 10.1007/BF01922330. [DOI] [PubMed] [Google Scholar]
- 29.Mansbach R S, Rovetti C C, Winston E N, Lowe J A. Psychopharmacology. 1996;124:315–322. doi: 10.1007/BF02247436. [DOI] [PubMed] [Google Scholar]
- 30.Randall M D, Kendall D A. Trends Pharmacol Sci. 1998;19:55–58. doi: 10.1016/s0165-6147(97)01161-9. [DOI] [PubMed] [Google Scholar]
- 31.Di Marzo V, Bisogno T, Melck D, Ross R, Brockie H, Stevenson L, Pertwee R, De Petrocellis L. FEBS Lett. 1998;436:449–454. doi: 10.1016/s0014-5793(98)01175-2. [DOI] [PubMed] [Google Scholar]
- 32.White R, Hiley R. Br J Pharmacol. 1997;122:1573–1584. doi: 10.1038/sj.bjp.0701546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zygmunt P M, Petersson J, Andersson D A, Chuang H, Sørgård M, Di Marzo V, Julius D, Högestätt E D. Nature (London) 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- 34.Gebremedhin D, Lange A R, Campbell W B, Hillard C J, Harder D R. Am J Physiol. 1999;276:H2085–H2093. doi: 10.1152/ajpheart.1999.276.6.H2085. [DOI] [PubMed] [Google Scholar]
- 35.Mombouli J-V, Schaeffer G, Holzmann S, Kostner G M, Graier W F. Br J Pharmacol. 1999;126:1593–1600. doi: 10.1038/sj.bjp.0702483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hampson A J, Grimaldi M, Axelrod J, Wink D. Proc Natl Acad Sci USA. 1998;95:8268–8273. doi: 10.1073/pnas.95.14.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCulloch A I, Bottrill F E, Randall D M, Hiley C R. Br J Pharmacol. 1997;120:1431–1438. doi: 10.1038/sj.bjp.0701066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zygmunt P M, Högestätt E D. Br J Pharmacol. 1996;117:1600–1606. doi: 10.1111/j.1476-5381.1996.tb15327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edwards G, Dora K A, Gardener M J, Garland C J, Weston A H. Nature (London) 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- 40.Campbell W B, Gebremedhin D, Pratt P F, Harder D R. Circ Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- 41.Newby A C, Henderson A H. Annu Rev Physiol. 1990;52:661–674. doi: 10.1146/annurev.ph.52.030190.003305. [DOI] [PubMed] [Google Scholar]
- 42.Fimiani C, Mattocks D, Cavani F, Salzet M, Deutsch D G, Pryor S, Bilfinger T V, Stefano B G. Cell Signal. 1999;11:189–193. doi: 10.1016/s0898-6568(98)00060-6. [DOI] [PubMed] [Google Scholar]
- 43.Deutsch D G, Goligorsky M S, Schmid P C, Krebsbach R J, Schmid H H, Das S K, Dey S K, Arreza G, Thorup C, Stefano G, Moore L C. J Clin Invest. 1997;100:1538–1546. doi: 10.1172/JCI119677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sugiura T, Kodaka T, Nakane S, Kishimoto S, Kondo S, Waku K. Biochem Biophys Res Commun. 1998;243:838–843. doi: 10.1006/bbrc.1998.8187. [DOI] [PubMed] [Google Scholar]