Abstract
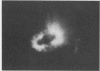
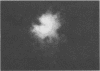
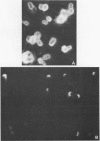
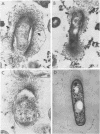
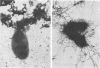
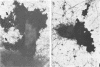
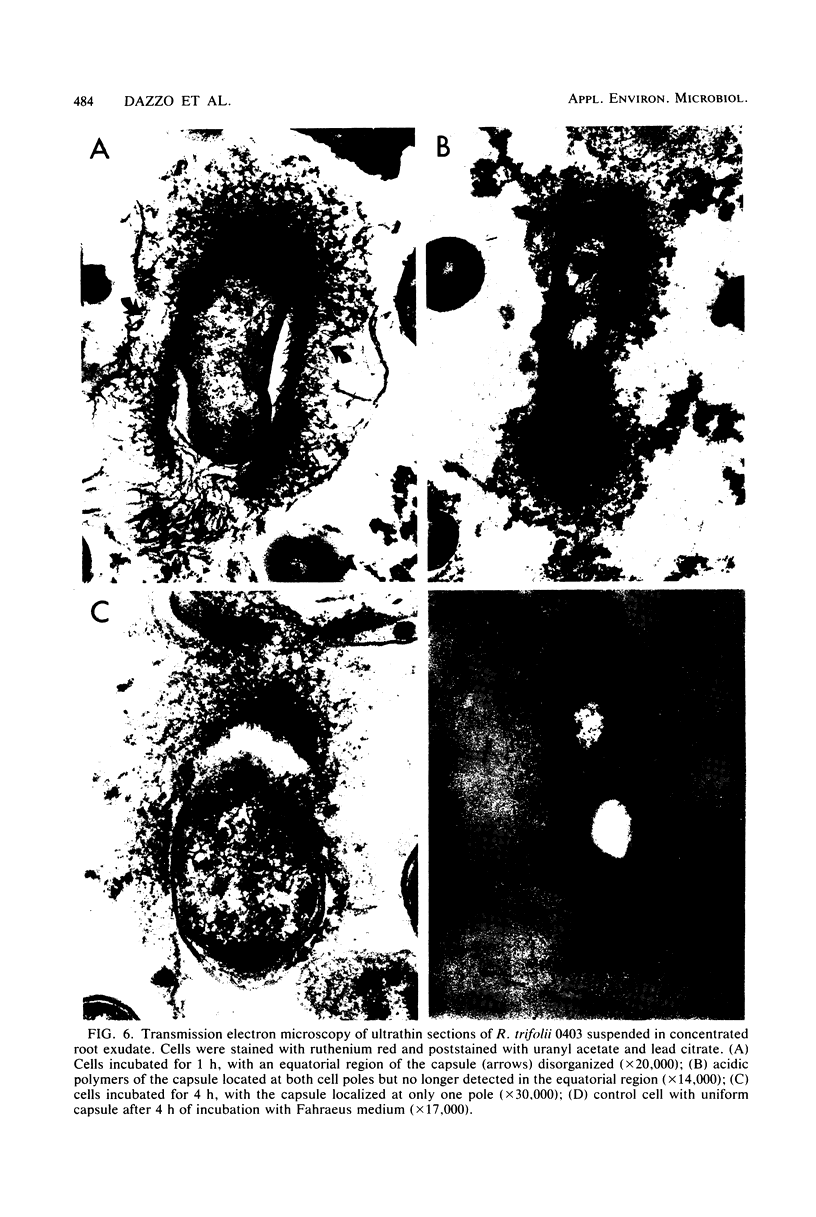
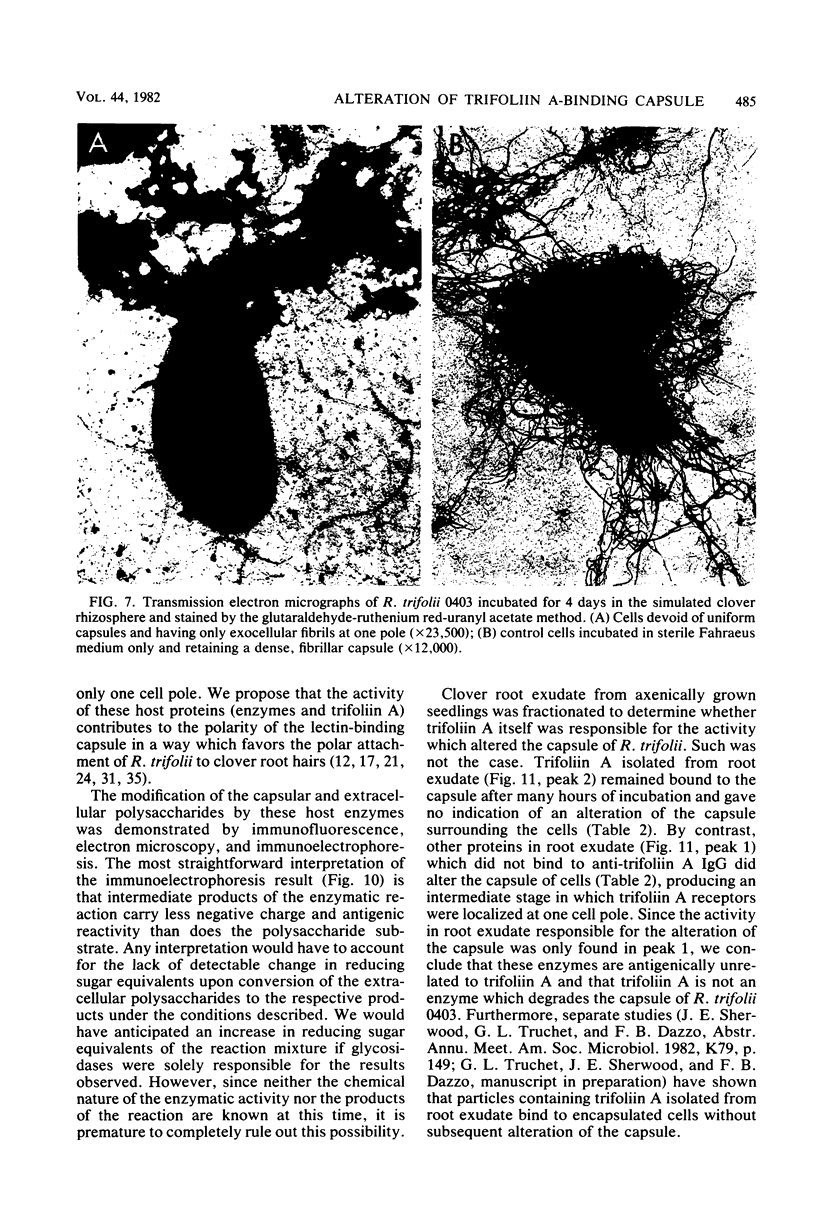
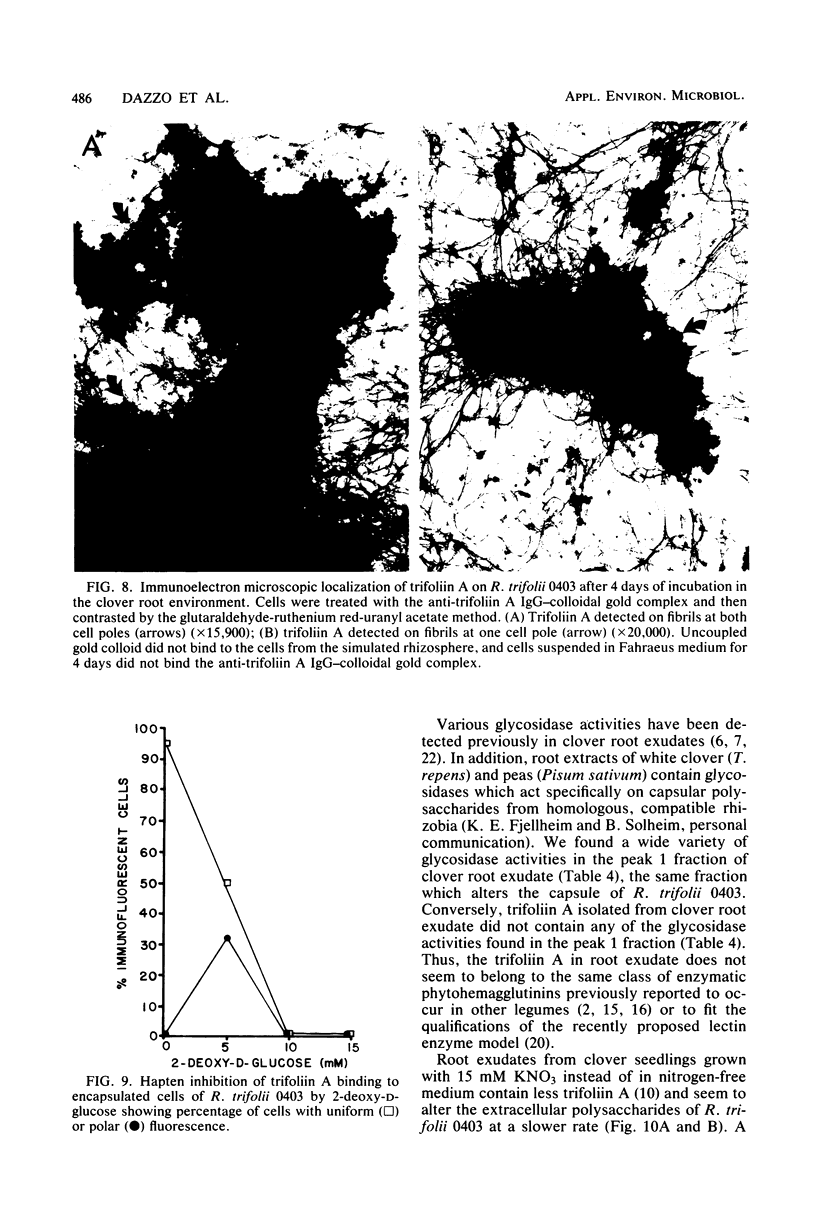
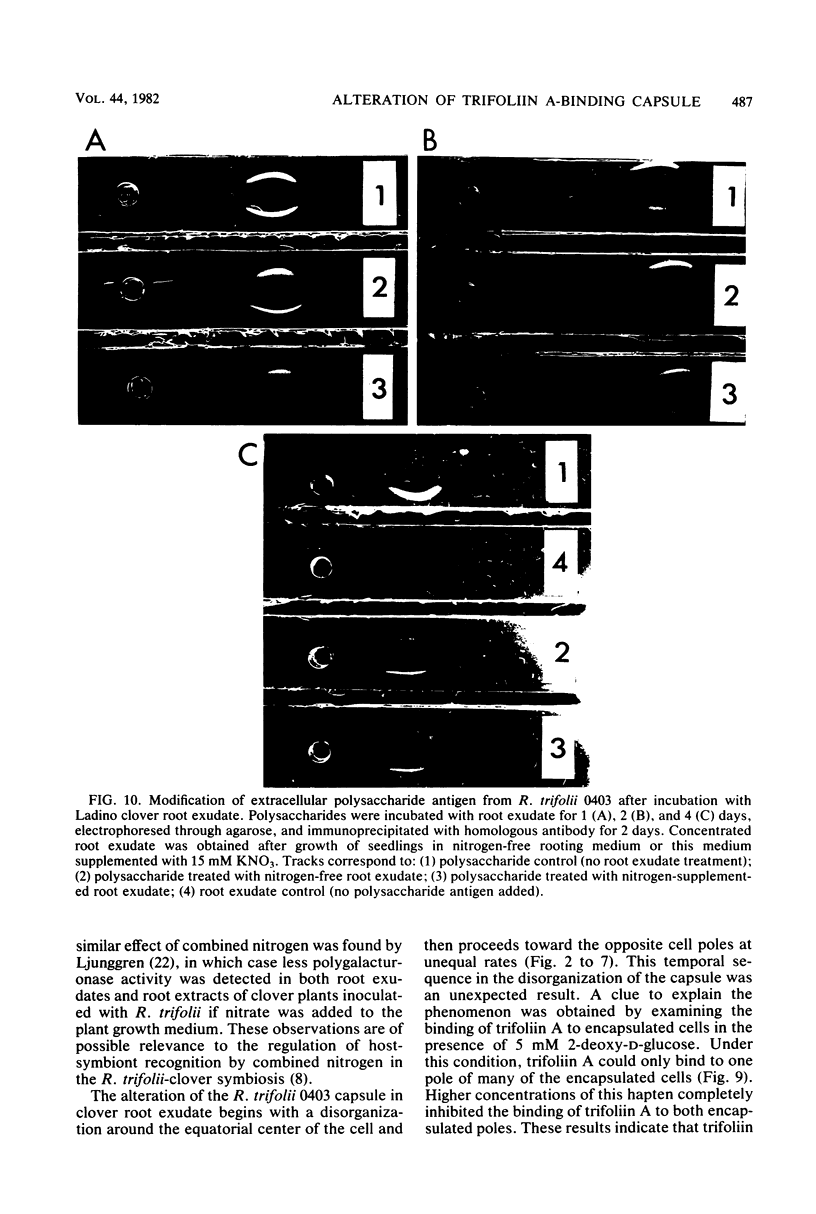
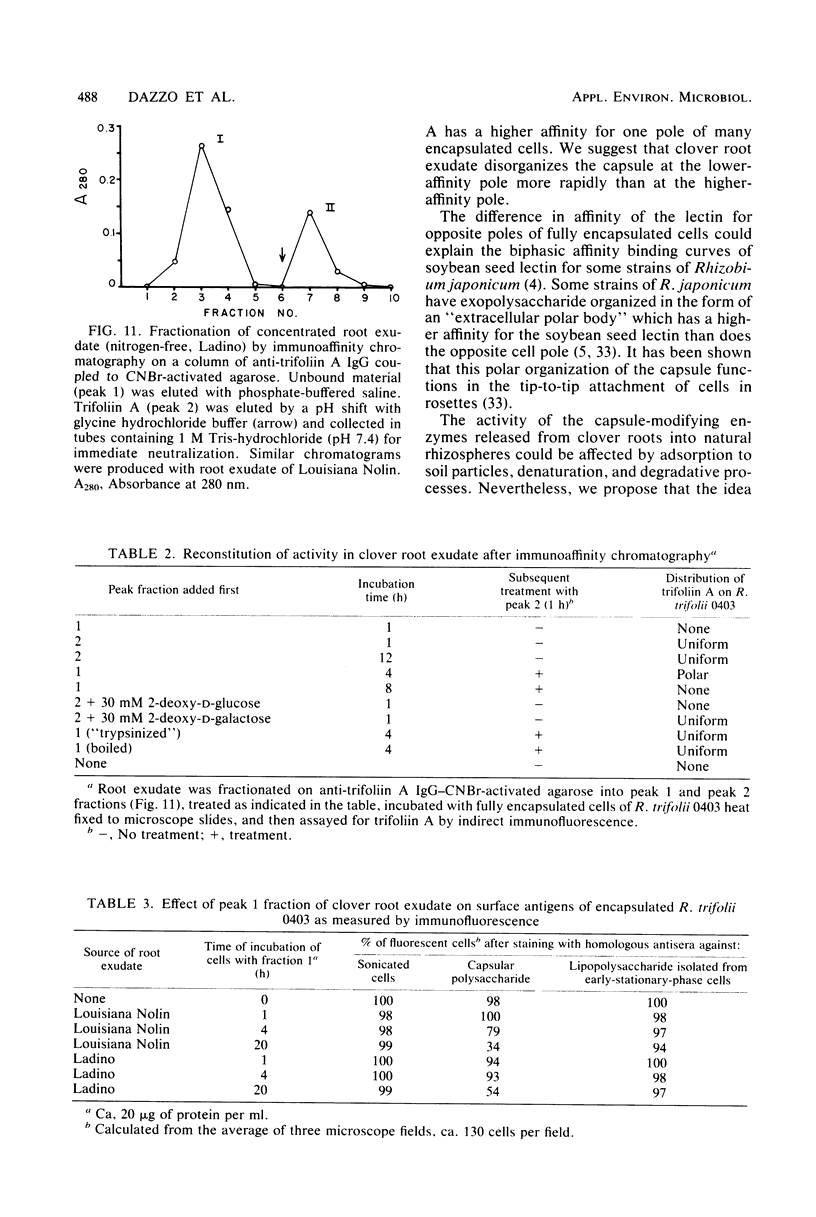
The effect of white clover root exudate on capsules of Rhizobium trifolii 0403 was examined. The clover lectin trifoliin A was detected in root exudate of two clover varieties by indirect immunofluorescence with antibody against this lectin purified from clover seed. Trifoliin A bound uniformly to encapsulated, heat-fixed cells during 1 h of incubation with root exudate. After 4 to 8 h of incubation, trifoliin A was only bound to one pole of the cells. Transmission electron microscopy showed that the capsule itself was altered. The disorganization of the acidic polymers of the capsule began in the equatorial center of the rod-shaped cell and then progressed toward the poles at unequal rates. Trifoliin A could no longer be detected on heat-fixed cells after 12 h of incubation with root exudate. However, trifoliin A was detected in situ on one pole of cells grown for 4 days in the clover root environment of Fahraeus slide cultures. Inhibition studies with the hapten 2-deoxy-d-glucose showed that trifoliin A in root exudate had a higher affinity for one of the cell poles. Immunoelectrophoresis was used to monitor the alteration of the extracellular polysaccharides from R. trifolii 0403 by concentrated root exudate. These polysaccharides were converted into products which eventually lost their ability to immunoprecipitate with homologous antibody. This progressive loss of antigenic reactivity proceeded more rapidly with root exudate from seedlings grown under nitrogen-free conditions than with root exudate from plants grown with 15 mM KNO3. The root exudate, depleted of trifoliin A by immunoaffinity chromatography, was still able to alter the capsule of R. trifolii 0403. Reconstitution experiments showed that the substance(s) in root exudate which induced this alteration of the capsule was of a high molecular weight, heat labile, trypsin sensitive, and antigenically unrelated to trifoliin A. A variety of glycosidase activities were also detected in the fraction depleted of trifoliin A. These results suggest that enzymes in clover root exudate alter the trifoliin A-binding capsule in a way which would favor polar attachment of R. trifolii to clover root hairs.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhagwat A. A., Thomas J. Legume-Rhizobium interactions: cowpea root exudate elicits faster nodulation response by Rhizobium species. Appl Environ Microbiol. 1982 Apr;43(4):800–805. doi: 10.1128/aem.43.4.800-805.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuvaneswari T. V., Pueppke S. G., Bauer W. D. Role of lectins in plant-microorganism interactions: I. Binding of soybean lectin to rhizobia. Plant Physiol. 1977 Oct;60(4):486–491. doi: 10.1104/pp.60.4.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlool B. B., Schmidt E. L. Immunofluorescent polar tips of Rhizobium japonicum: possible site of attachment or lectin binding. J Bacteriol. 1976 Mar;125(3):1188–1194. doi: 10.1128/jb.125.3.1188-1194.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Brill W. J. Bacterial polysaccharide which binds Rhizobium trifolii to clover root hairs. J Bacteriol. 1979 Mar;137(3):1362–1373. doi: 10.1128/jb.137.3.1362-1373.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Brill W. J. Regulation by fixed nitrogen of host-symbiont recognition in the Rhizobium-clover symbiosis. Plant Physiol. 1978 Jul;62(1):18–21. doi: 10.1104/pp.62.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Hrabak E. M. Presence of trifoliin A, a Rhizobium-binding lectin, in clover root exudate. J Supramol Struct Cell Biochem. 1981;16(2):133–138. doi: 10.1002/jsscb.1981.380160204. [DOI] [PubMed] [Google Scholar]
- Dazzo F. B., Hubbell D. H. Antigenic differences between infective and noninfective strains of Rhizobium trifolii. Appl Microbiol. 1975 Aug;30(2):172–177. doi: 10.1128/am.30.2.172-177.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Napoli C. A., Hubbell D. H. Adsorption of bacteria to roots as related to host specificity in the Rhizobium-clover symbiosis. Appl Environ Microbiol. 1976 Jul;32(1):166–171. doi: 10.1128/aem.32.1.166-171.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Yanke W. E., Brill W. J. Trifolin: a Rhizobium recognition protein from white clover. Biochim Biophys Acta. 1978 Mar 20;539(3):276–286. doi: 10.1016/0304-4165(78)90032-6. [DOI] [PubMed] [Google Scholar]
- Del Campillo E., Shannon L. M. An alpha-Galactosidase with Hemagglutinin Properties from Soybean Seeds. Plant Physiol. 1982 Mar;69(3):628–631. doi: 10.1104/pp.69.3.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAHRAEUS G. The infection of clover root hairs by nodule bacteria studied by a simple glass slide technique. J Gen Microbiol. 1957 Apr;16(2):374–381. doi: 10.1099/00221287-16-2-374. [DOI] [PubMed] [Google Scholar]
- Hankins C. N., Kindinger J. I., Shannon L. M. Legume alpha-Galactosidases Which Have Hemagglutinin Properties. Plant Physiol. 1980 Apr;65(4):618–622. doi: 10.1104/pp.65.4.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankins C. N., Shannon L. M. The physical and enzymatic properties of a phytohemagglutinin from mung beans. J Biol Chem. 1978 Nov 10;253(21):7791–7797. [PubMed] [Google Scholar]
- Higashi S., Abe M. Scanning Electron Microscopy of Rhizobium trifolii Infection Sites on Root Hairs of White Clover. Appl Environ Microbiol. 1980 Dec;40(6):1094–1099. doi: 10.1128/aem.40.6.1094-1099.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horisberger M., Rosset J., Bauer H. Colloidal gold granules as markers for cell surface receptors in the scanning electron microscope. Experientia. 1975 Oct 15;31(10):1147–1149. doi: 10.1007/BF02326761. [DOI] [PubMed] [Google Scholar]
- Hrabak E. M., Urbano M. R., Dazzo F. B. Growth-phase-dependent immunodeterminants of Rhizobium trifolii lipopolysaccharide which bind trifoliin A, a white clover lectin. J Bacteriol. 1981 Nov;148(2):697–711. doi: 10.1128/jb.148.2.697-711.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marshall K. C., Cruickshank R. H., Bushby H. V. The orientation of certain root-nodule bacteria at interfaces, including legume root-hair surfaces. J Gen Microbiol. 1975 Nov;91(1):198–200. doi: 10.1099/00221287-91-1-198. [DOI] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate J. L., Ordal E. J. The fine structure of Chondrococcus columnaris. 3. The surface layers of Chondrococcus columnaris. J Cell Biol. 1967 Oct;35(1):37–51. doi: 10.1083/jcb.35.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAHLMAN K. AN ELECTRON MICROSCOPE STUDY OF ROOT-HAIR INFECTION BY RHIZOBIUM. J Gen Microbiol. 1963 Dec;33:425–427. doi: 10.1099/00221287-33-3-425. [DOI] [PubMed] [Google Scholar]
- Tsien H. C., Schmidt E. L. Polarity in the exponential-phase Rhizobium japonicum cell. Can J Microbiol. 1977 Sep;23(9):1274–1284. doi: 10.1139/m77-191. [DOI] [PubMed] [Google Scholar]
- Wright B. G., Rebers P. A. Procedure for determining heptose and hexose in lipopolysaccharides. Modification of the cysteine-sulfuric acid method. Anal Biochem. 1972 Oct;49(2):307–319. doi: 10.1016/0003-2697(72)90433-2. [DOI] [PubMed] [Google Scholar]
- Zurkowski W. Specific adsorption of bacteria to clover root hairs, related to the presence of the plasmid pWZ2 in cells of Rhizobium trifolii. Microbios. 1980;27(107):27–32. [PubMed] [Google Scholar]