Abstract
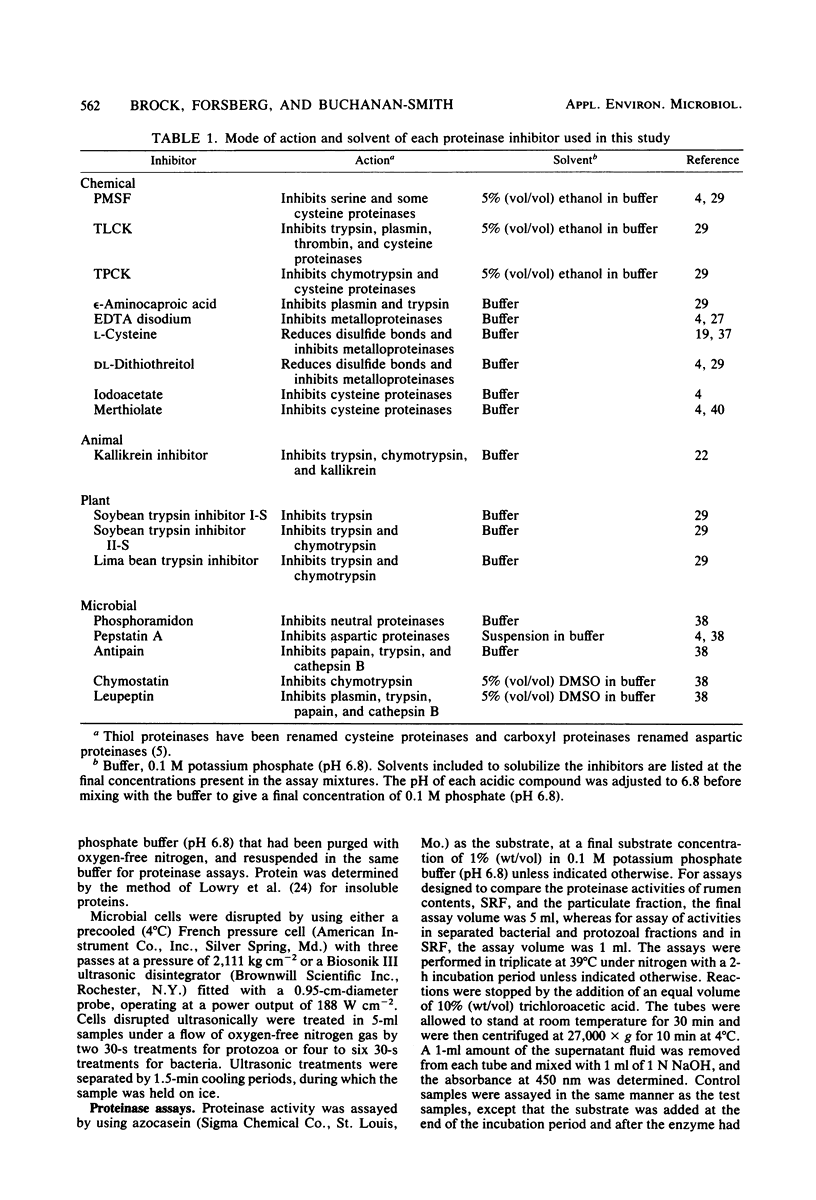
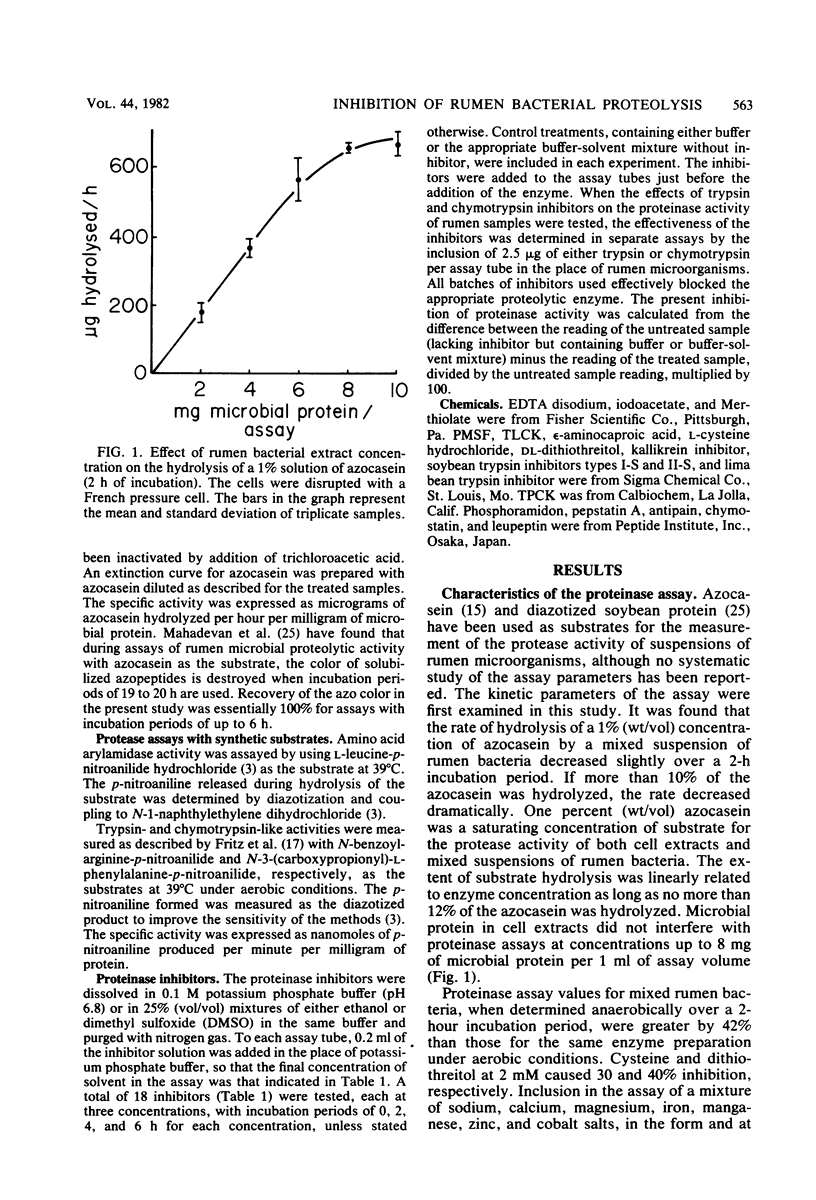
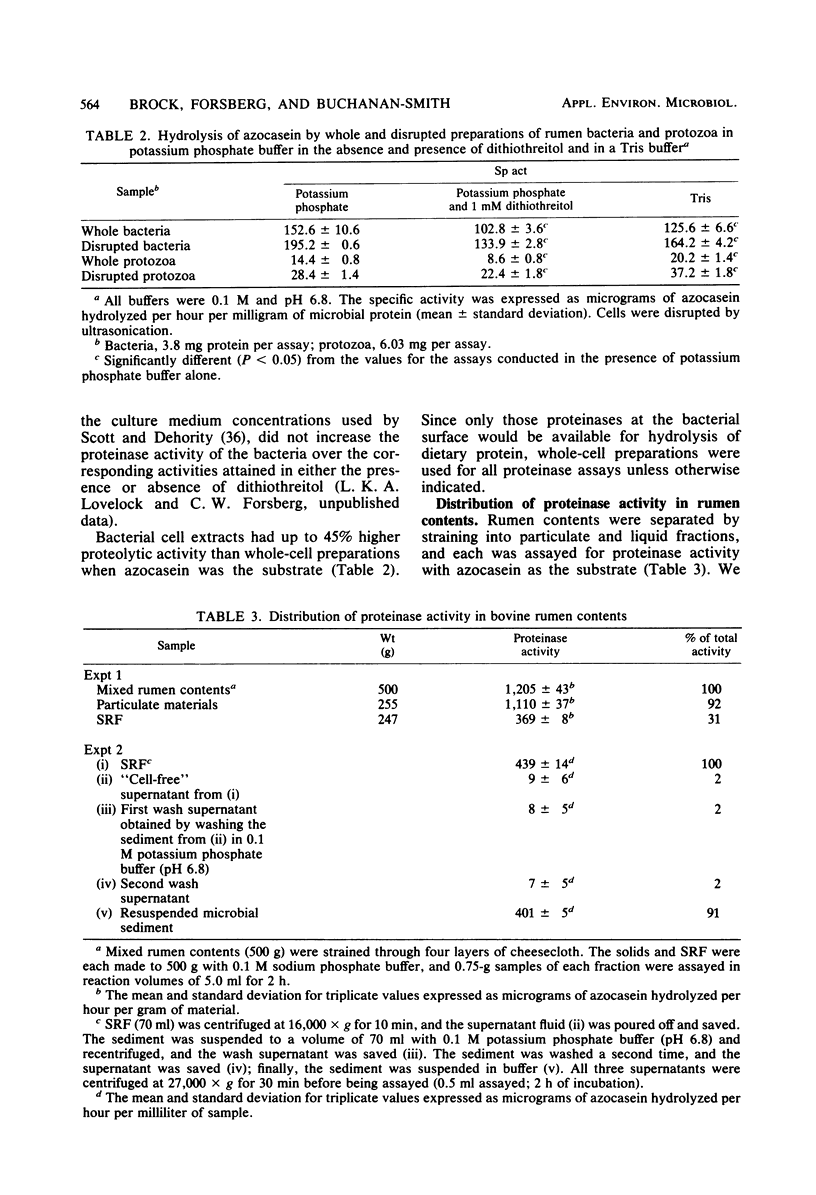
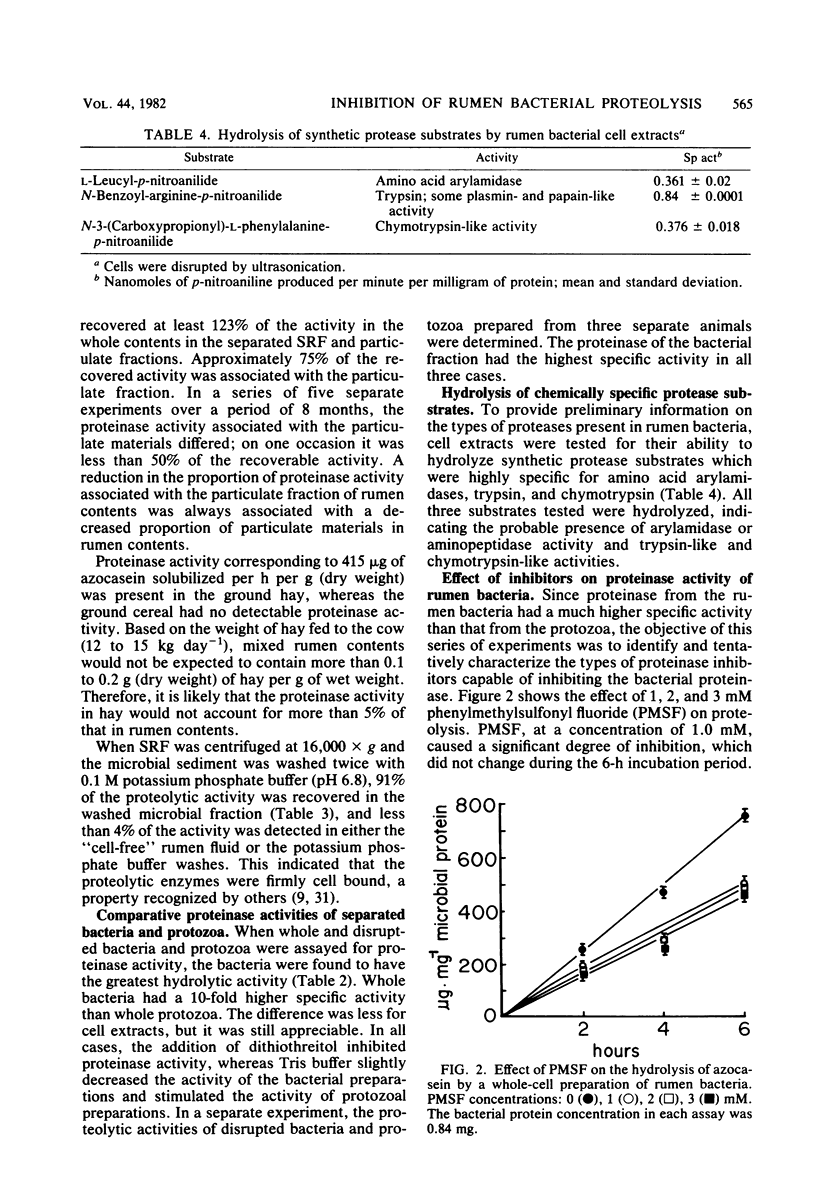
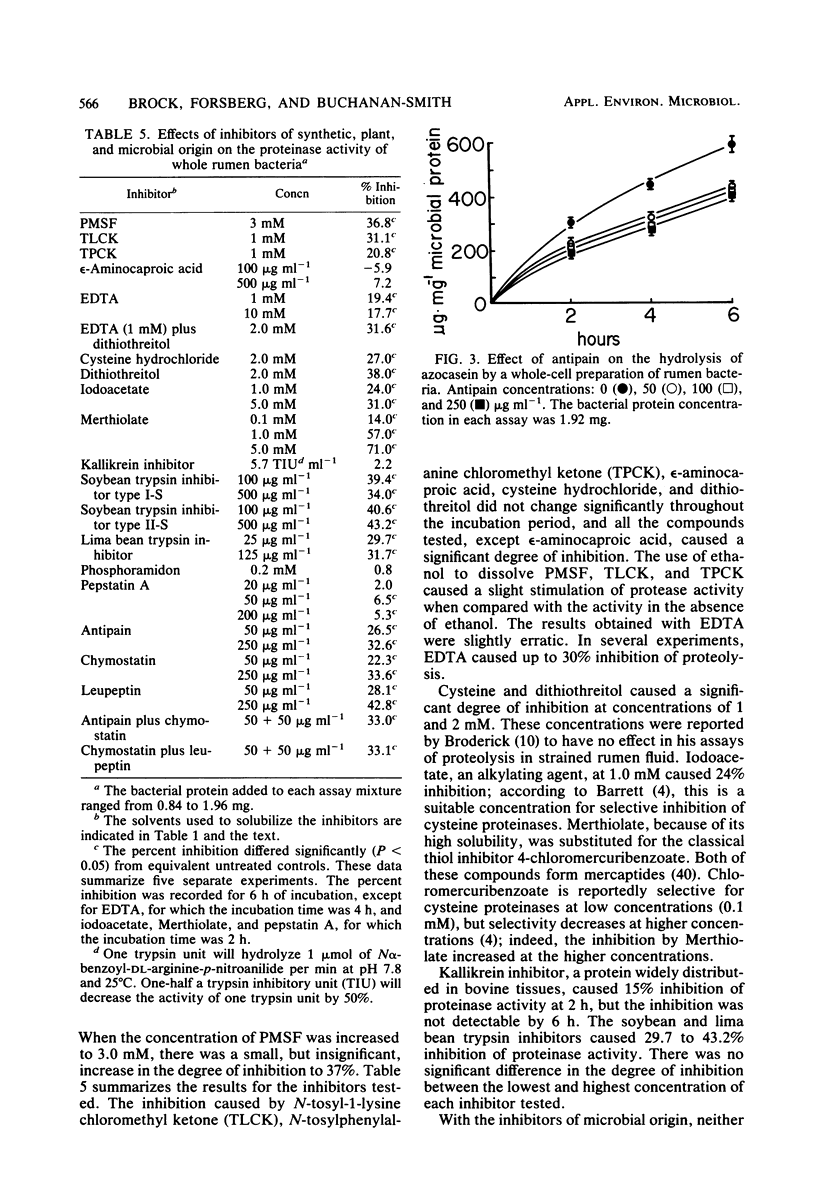
Proteolytic activity of the bovine rumen microflora was studied with azocasein as the substrate. Approximately 25% of the proteolytic activity of rumen contents was recovered in the strained rumen fluid fraction, and the balance of the activity was associated with the particulate fraction. The proportion of proteinase activity associated with particulate material decreased when the quantity of particulate material in rumen contents was reduced. The specific activity of the proteinase from the bacterial fraction was 6 to 10 times higher than that from the protozoal fraction. Proteinase inhibitors of synthetic, plant, and microbial origin were tested on proteolytic activity of the separated bacteria. Synthetic proteinase inhibitors that caused significant inhibition of proteolysis included phenylmethylsulfonyl fluoride, N-tosyl-1-lysine chloromethyl ketone, N-tosylphenylalanine chloromethyl ketone, EDTA, cysteine, dithiothreitol, iodoacetate, and Merthiolate. Plant proteinase inhibitors that had an inhibitory effect included soybean trypsin inhibitors types I-S and II-S and the lima bean trypsin inhibitor. Proteinase inhibitors of microbial origin that showed an inhibitory effect included antipain, leupeptin, and chymostatin; phosphoramidon and pepstatin had little effect. We tentatively concluded that rumen bacteria possess, primarily, serine, cysteine, and metalloproteinases.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABOU AKKADA A. R., HOWARD B. H. The biochemistry of rumen protozoa. 5. The nitrogen metabolism of Entodinium. Biochem J. 1962 Feb;82:313–320. doi: 10.1042/bj0820313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbey B. W., Norton G., Neale R. J. Effects of dietary proteinase inhibitors from field bean (Vicia faba L.) and field-bean meal on pancreatic function in the rat. Br J Nutr. 1979 Jan;41(1):39–45. doi: 10.1079/bjn19790009. [DOI] [PubMed] [Google Scholar]
- BLACKBURN T. H., HOBSON P. N. Proteolysis in the sheep rumen by whole and fractionated rumen contents. J Gen Microbiol. 1960 Feb;22:272–281. doi: 10.1099/00221287-22-1-272. [DOI] [PubMed] [Google Scholar]
- Barrett A. J. Protein degradation in health and disease. Introduction: the classification of proteinases. Ciba Found Symp. 1979;(75):1–13. [PubMed] [Google Scholar]
- Bird S. H., Hill M. K., Leng R. A. The effects of defaunation of the rumen on the growth of lambs on low-protein-high-energy diets. Br J Nutr. 1979 Jul;42(1):81–87. doi: 10.1079/bjn19790091. [DOI] [PubMed] [Google Scholar]
- Blackburn T. H. The protease liberated from Bacteroides amylophilus strain H18 by mechanical disintegration. J Gen Microbiol. 1968 Aug;53(1):37–51. doi: 10.1099/00221287-53-1-37. [DOI] [PubMed] [Google Scholar]
- Broderick G. A. In vitro procedures for estimating rates of ruminal protein degradation and proportions of protein escaping the rumen undegraded. J Nutr. 1978 Feb;108(2):181–190. doi: 10.1093/jn/108.2.181. [DOI] [PubMed] [Google Scholar]
- Chalupa W. Degradation of amino acids by the mixed rumen microbial population. J Anim Sci. 1976 Oct;43(4):828–834. doi: 10.2527/jas1976.434828x. [DOI] [PubMed] [Google Scholar]
- Chalupa W. Rumen bypass and protection of proteins and amino acids. J Dairy Sci. 1975 Aug;58(8):1198–1218. doi: 10.3168/jds.S0022-0302(75)84697-2. [DOI] [PubMed] [Google Scholar]
- Coleman G. S. Rumen ciliate protozoa. Adv Parasitol. 1980;18:121–173. doi: 10.1016/s0065-308x(08)60399-1. [DOI] [PubMed] [Google Scholar]
- Dinsdale D., Cheng K. J., Wallace R. J., Goodlad R. A. Digestion of epithelial tissue of the rumen wall by adherent bacteria in infused and conventionally fed sheep. Appl Environ Microbiol. 1980 May;39(5):1059–1066. doi: 10.1128/aem.39.5.1059-1066.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FULGHUM R. S., MOORE W. E. ISOLATION, ENUMERATION, AND CHARACTERISTICS OF PROTEOLYTIC RUMINAL BACTERIA. J Bacteriol. 1963 Apr;85:808–815. doi: 10.1128/jb.85.4.808-815.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. W. Some effects of arsenic on the rumen microflora; an in vitro study. Can J Microbiol. 1978 Jan;24(1):36–44. doi: 10.1139/m78-007. [DOI] [PubMed] [Google Scholar]
- Hazlewood G. P., Edwards R. Proteolytic activities of a rumen bacterium, Bacteroides ruminicola R8/4. J Gen Microbiol. 1981 Jul;125(1):11–15. doi: 10.1099/00221287-125-1-11. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mangan J. L. Quantitative studies on nitrogen metabolism in the bovine rumen. The rate of proteolysis of casein and ovalbumin and the release and metabolism of free amino acids. Br J Nutr. 1972 Mar;27(2):261–283. doi: 10.1079/bjn19720092. [DOI] [PubMed] [Google Scholar]
- Nugent J. H., Mangan J. L. Characteristics of the rumen proteolysis of fraction I (18S) leaf protein from lucerne (Medicago sativa L). Br J Nutr. 1981 Jul;46(1):39–58. doi: 10.1079/bjn19810007. [DOI] [PubMed] [Google Scholar]
- Poos M. I., Hanson T. L., Klopfenstein T. J. Monensin effects on diet digestibility, ruminal protein bypass and microbial protein synthesis. J Anim Sci. 1979 Jun;48(6):1516–1524. doi: 10.2527/jas1979.4861516x. [DOI] [PubMed] [Google Scholar]
- Prins R. A., van Hal-Van Gestel J. C., Counotte G. H. Degradation of amino acids and peptides by mixed rumen micro-organisms. Z Tierphysiol Tierernahr Futtermittelkd. 1979 Dec;42(6):333–339. doi: 10.1111/j.1439-0396.1979.tb01226.x. [DOI] [PubMed] [Google Scholar]
- Russell J. B., Bottje W. G., Cotta M. A. Degradation of protein by mixed cultures of rumen bacteria: identification of Streptococcus bovis as an actively proteolytic rumen bacterium. J Anim Sci. 1981 Jul;53(1):242–252. doi: 10.2527/jas1981.531242x. [DOI] [PubMed] [Google Scholar]
- SCOTT H. W., DEHORITY B. A. VITAMIN REQUIREMENTS OF SEVERAL CELLULOLYTIC RUMEN BACTERIA. J Bacteriol. 1965 May;89:1169–1175. doi: 10.1128/jb.89.5.1169-1175.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker-Simmons M., Ryan C. A. Wound-induced Accumulation of Trypsin Inhibitor Activities in Plant Leaves: Survey of Several Plant Genera. Plant Physiol. 1977 Mar;59(3):437–439. doi: 10.1104/pp.59.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]


