Abstract
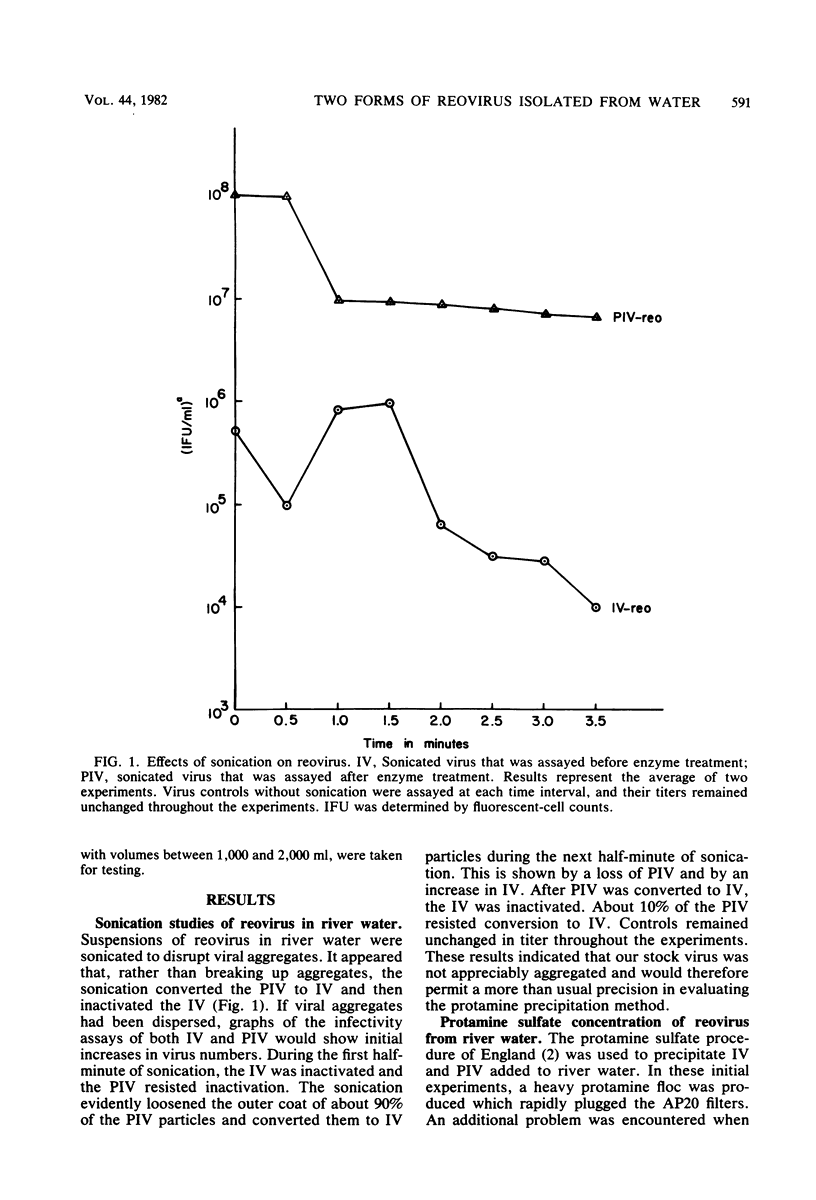
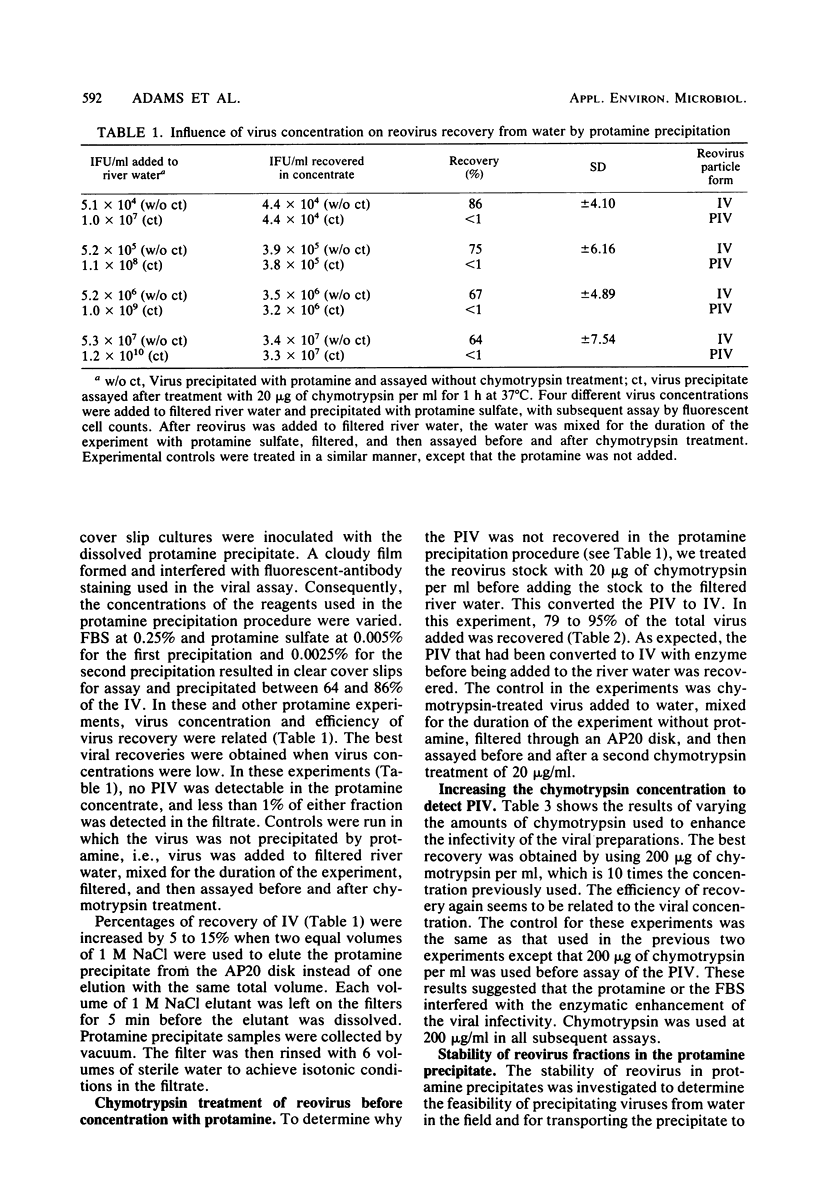
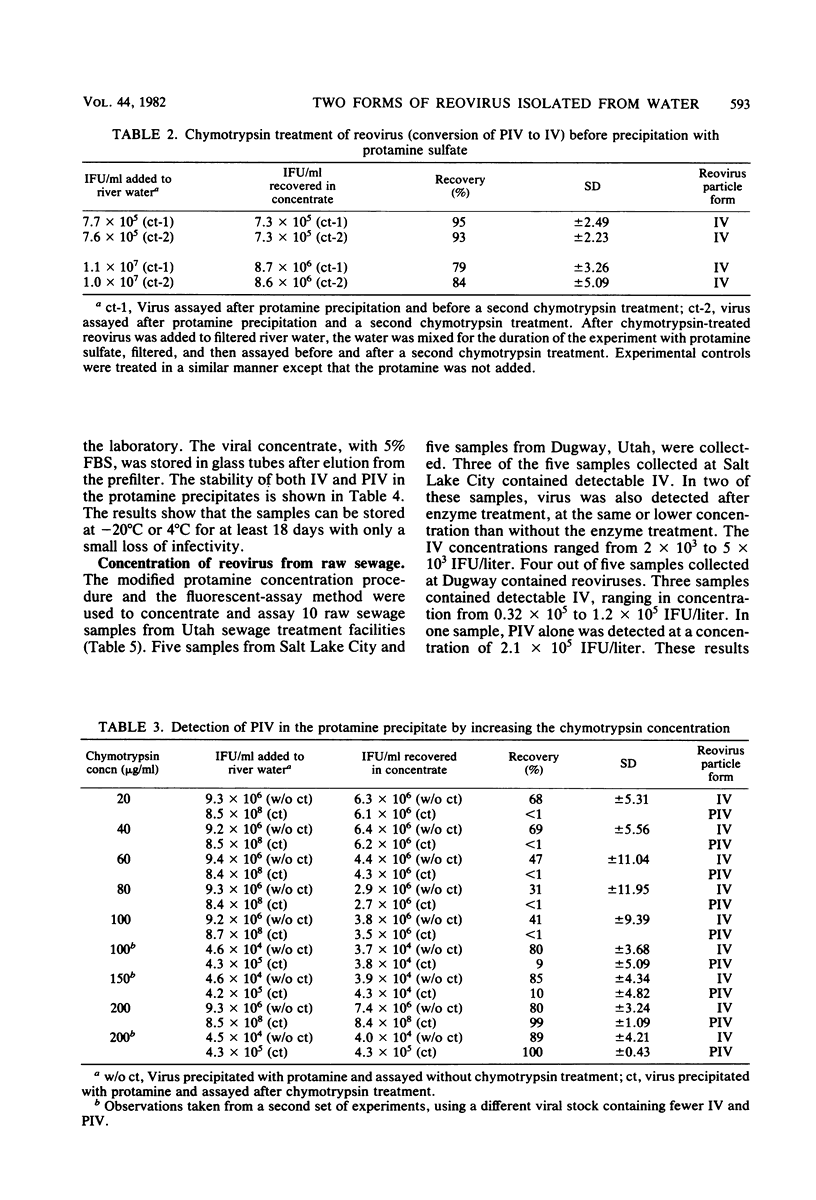
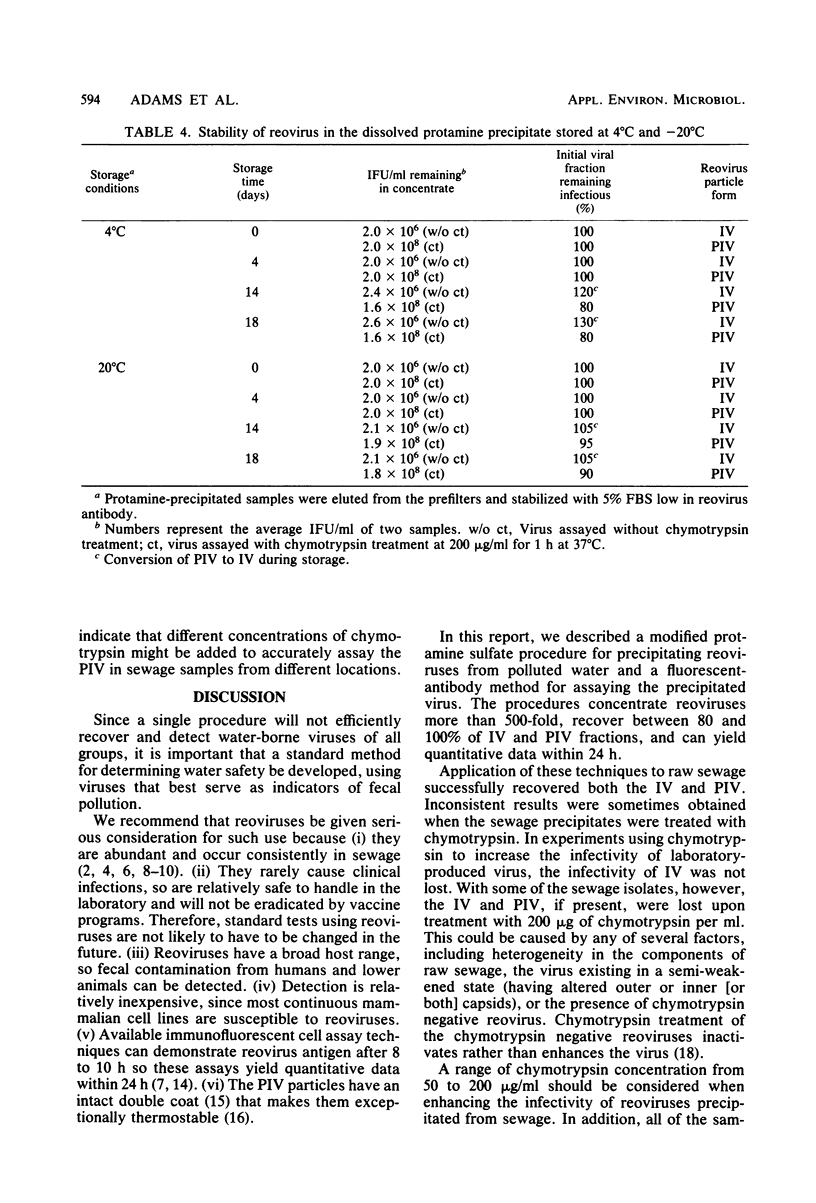
Two forms of virus particle are released from reovirus-infected cell cultures, infectious reovirus and potentially infectious reovirus (PIV). PIV particle forms have a complete outer coat and are not infectious until the outer coat is altered or removed. The PIV concentration in polluted waters, however, has not been determined. Protamine sulfate precipitation, using 0.25% fetal bovine serum and 0.005% protamine sulfate for the first precipitation of the sample and 0.0025% for the second, was employed to concentrate infectious reovirus and PIV from water and sewage. Infectious reovirus and PIV particles were concentrated over 500-fold from river water inoculated with virus, and virus recoveries of between 80 and 100% were achieved. Virus precipitates stored at -20 degrees C as a protamine-virus concentrate showed a 5% loss of PIV after 14 days. Virus preparations were assayed, before and after treatment, with 200 micrograms of chymotrypsin per ml, using a fluorescent-antibody procedure. Protamine sulfate precipitation and fluorescent-antibody detection are effective ways to recover and assay reoviruses present in raw sewage.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cliver D. O. Virus association with wastewater solids. Environ Lett. 1975;10(3):215–223. doi: 10.1080/00139307509435823. [DOI] [PubMed] [Google Scholar]
- England B. Concentration of reovirus and adenovirus from sewage and effluents by protamine sulfate (salmine) treatment. Appl Microbiol. 1972 Sep;24(3):510–512. doi: 10.1128/am.24.3.510-512.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd R., Sharp D. G. Aggregation of poliovirus and reovirus by dilution in water. Appl Environ Microbiol. 1977 Jan;33(1):159–167. doi: 10.1128/aem.33.1.159-167.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jopkiewicz T., Krzemińska K., Stachowska Z. Przeglad wirusologiczny ścieków z terenu miasta Bydgoszczy. I. Przegl Epidemiol. 1968;22(4):521–527. [PubMed] [Google Scholar]
- Knocke K. W., Pittler H., Höpken W. Nachweis von Reo-Viren in Abwässern. Zentralbl Bakteriol Orig. 1967;203(4):417–421. [PubMed] [Google Scholar]
- McClain M. E., Spendlove R. S., Lennette E. H. Infectivity assay of Reoviruses: comparison of immunofluorescent cell count and plaque methods. J Immunol. 1967 Jun;98(6):1301–1308. [PubMed] [Google Scholar]
- Pálfi A. B. Virus content of sewage in different seasons in Hungary. Acta Microbiol Acad Sci Hung. 1971;18(4):231–237. [PubMed] [Google Scholar]
- SPENDLOVE R. S., LENNETTE E. H., CHIN J. N., KNIGHT C. O. EFFECT OF ANTIMITOTIC AGENTS ON INTRACELLULAR REOVIRUS ANTIGEN. Cancer Res. 1964 Nov;24:1826–1833. [PubMed] [Google Scholar]
- SPENDLOVE R. S., SCHAFFER F. L. ENZYMATIC ENHANCEMENT OF INFECTIVITY OF REOVIRUS. J Bacteriol. 1965 Mar;89:597–602. doi: 10.1128/jb.89.3.597-602.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattar S., Westwood J. C. Viral pollution of surface waters due to chlorinated primary effluents. Appl Environ Microbiol. 1978 Sep;36(3):427–431. doi: 10.1128/aem.36.3.427-431.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spendlove R. S., Lennette E. H., Knight C. O., Chin J. N. Production in FL cells of infectious and potentially infectious reovirus. J Bacteriol. 1966 Oct;92(4):1036–1040. doi: 10.1128/jb.92.4.1036-1040.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spendlove R. S., McClain M. E., Lennette E. H. Enhancement of reovirus infectivity by extracellular removal or alteration of the virus capsid by proteolytic enzymes. J Gen Virol. 1970 Aug;8(2):83–94. doi: 10.1099/0022-1317-8-2-83. [DOI] [PubMed] [Google Scholar]
- Ward R. L., Ashley C. S. Identification of the virucidal agent in wastewater sludge. Appl Environ Microbiol. 1977 Apr;33(4):860–864. doi: 10.1128/aem.33.4.860-864.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


