Abstract
The cells of the endosperm of castor bean seeds (Ricinus communis) undergo programmed cell death during germination, after their oil and protein reserves have been mobilized. Nuclear DNA fragmentation first was observed at day 3 in the endosperm cells immediately adjacent to the cotyledons and progressed across to the outermost cell layers by day 5. We also detected the accumulation of small organelles known as ricinosomes, by using an antibody against a cysteine endoprotease. By the time the nuclear DNA was susceptible to heavy label by terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling, the ricinosomes had released into the cytoplasm their content of cysteine endoprotease, which became activated because of the cleavage of its propeptide. The cysteine endoprotease is distinguished by a C-terminal KDEL sequence, although it is not retained in the lumen of the endoplasmic reticulum and is a marker for ricinosomes. Homologous proteases are found in the senescing tissues of other plants, including the petals of the daylily. Ricinosomes were identified in this tissue by electron microscopy and immunocytochemistry. It seems that ricinosomes are not unique to Ricinus and play an important role in the degradation of plant cell contents during programmed cell death.
Keywords: Ricinus communis, Hemerocallissp., papain-type KDEL peptidase
Programmed cell death (PCD) is a physiological process involved in the selective elimination of unwanted cells (1). This orderly process, also known as apoptosis, depends on the active participation of the dying cell. Functionally, PCD differs from degenerative death or necrosis in its nature and biological significance (2). PCD occurs as part of normal development, as well as in pathological processes associated with some diseases (2, 3). In plants, PCD is essential and occurs during pathogenesis, xylogenesis, reproduction, and senescence (4). A comparison of PCD in plants and animals reveals obvious similarities. In particular, DNA is degraded and cysteine proteases are expressed or activated (5, 6). Fragmentation of the nucleus appears to be a hallmark of apoptosis, and the responsible endonucleases may be activated indirectly by proteases that act as apoptosis regulators (7). Most animal cysteine proteases associated with apoptosis cleave after an aspartic acid residue (8) and therefore have been termed caspases. Caspases function in an activation cascade and are constitutively present in most cells, residing in the cytosol as a single chain proenzyme (6). A protease cascade as a regulator of PCD has not yet been described in plants. However, cysteine proteases are induced in plant systems undergoing PCD, such as tracheary element differentiation in Zinnia elegans (9, 10) and soybean cells exposed to oxidative stress (11).
Senescence is one of the processes described as PCD (4). It is the final phase of plant vegetative and reproductive development, preceding the widespread death of cells and organs. Senescence involves the active turnover and recapture of cellular material for use in other organs. Several senescing tissues in plants express a subgroup of the papain-type cysteine endoprotease that are distinguished by a C-terminal KDEL motif. Members of this subgroup were identified in senescing flowers of daylily (Hemerocallis) (12) and Christmas bells (Sandersonia aurantiaca; AF133839). They are expressed in the outer integument of Phalaenopsis in late stages of ovule development (13). A similar endoprotease (EP-C1) is found in late stages of fruit maturation of Phaseolus vulgaris in ripening pods and is thought to be responsible for the degradation of proteins to be translocated as amino acids to developing seeds (14). A homologous thiol-protease is induced during ovary senescence in Pisum sativum (15). A cysteine endoprotease (EP-A) with a C-terminal -TDEL motif (Z97023) is secreted from barley aleurone cells during germination. Aleurone cells are unnecessary for postembryonic development and die as soon as germination is complete (16).
The cotyledons of the hypogaeic Vicia sativa and P. sativum die during germination as soon as the storage material has been mobilized. In V. sativa, proteinase A, a papain-like cysteine endoprotease was detected in cotyledon extracts of 6-day germinated seeds when globulin degradation had already proceeded to a large extent (17). The cotyledons of the epigaeic Vigna mungo do not become green during germination; they are a senescing tissue and abscise after storage mobilization. A cysteine endoprotease (SH-EP) was synthesized only in the cotyledons during germination and the amount of the enzyme increased until day 4 after imbibition. (18).
Castor beans store oil and proteins in a living endosperm laterally attached to the cotyledons. From days 1 to 5 of germination, glyoxysomes develop rapidly in the endosperm cells and mobilize triglycerides of the oleosomes. On day 6, when the oil reserves are depleted, the endosperm becomes increasingly slimy and detaches from the cotyledons. Endosperm tissue from Ricinus contains an additional organelle, only slightly smaller than glyoxysomes. This organelle was discovered in ultrastructural and cytochemical studies by two groups and was called ricinosome, because it was found in castor bean (19), or dilated cisternae, because it seemed to develop from the endoplasmic reticulum (ER) (20). We have purified and cloned a papain-type castor bean cysteine endoprotease (Cys-EP) with a molecular mass of 35 kDa for the mature enzyme that cleaves C-terminal to arginine (21). This Cys-EP is as a marker enzyme for ricinosomes (22). The Cys-EP precursor protein exhibits a presequence for cotranslational targeting into the ER, an N-terminal propeptide, a C-terminal KDEL motif, and a very high homology to the cysteine proteases found in the above-mentioned senescing tissues (22).
In this paper, we show that nuclear DNA fragmentation parallels the development of ricinosomes, which accumulate the 45-kDa proenzyme form of the Cys-EP in the senescing cells of germinating Ricinus endosperm. In the final stage of senescence, the ricinosomes disintegrate, releasing the mature 35-kDa form of Cys-EP. Furthermore, we found a similar cysteine endoprotease-containing organelle in senescing Hemerocallis flowers. We propose that ricinosomes play a special role in the final stage of senescence by releasing a cysteine endopeptidase for the breakdown of cellular protein for use in other organs.
Materials and Methods
Plant Materials.
Castor bean seeds (Ricinus communis L.) were soaked in running tap water overnight and grown in moist autoclaved vermiculite in the dark at 30°C for 2–6 days. Daylily bulbs (Hemerocallis) were put into soil in late fall and grown under natural conditions. Petals were harvested approximately 8 h after flowering.
Biochemical Procedures.
Protein extracts were prepared by grinding tissue in liquid nitrogen and extracting soluble proteins in buffer [50 mM Tris⋅HCl, pH 7.5/5 mM EDTA/250 μM PMSF/25 μM chymostatin/5 μM N-(trans-epoxysuccinyl)-l-leucine-4-guanidino-butylamide (E64)/1 μM leupeptin/1 μM pepstatin A] for 15 min at 4°C. SDS/PAGE was performed according to Laemmli (23) on 12% acrylamide gels. After electroblotting on nitrocellulose, blots were blocked with 1% (wt/vol) BSA in Tris-buffered saline (TBS; 150 mM NaCl) for 1 h at room temperature and incubated with primary antibody raised against the purified 35-kDa Cys-EP (diluted 1:1,000 in blocking solution) for an additional hour. Blots were washed with TBS containing 0.05% (vol/vol) Tween-20 and TBS 3 × 5 min each, and immunoreactive proteins were visualized by using alkaline phosphatase-coupled goat anti-rabbit IgG with nitro blue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate as a substrate.
Cloning, Expression, Purification, and Production of a Specific Antibody Against the Endopeptidase Propeptide.
A 315-bp fragment encoding the propeptide of the castor bean endopeptidase (22) was amplified from the cDNA by PCR, changing F21M for the translation start. The PCR product was cloned into the pQE60 vector (Qiagen, Chatsworth, CA) in-frame to the C-terminal 6× His tag. Production of the propeptide was induced by 1 mM isopropyl β-d-thiogalactopyranoside, and the protein was purified by using Talon metal affinity resin (CLONTECH) according to the manufacturer’s instructions. The protein was further purified by SDS/PAGE, and immunization of rabbits was carried out by using the antigen in acrylamide (Eurogentec, Brussels). The specificity of the anti-propeptide antibody was checked by Western blot analysis (data not shown).
Electron Microscopy (EM) and Immunocytochemistry.
Tissue was fixed, embedded, and stained as described (22), but castor bean endosperm was dehydrated in an ethanol series instead of chemical dehydration with acidified dimethoxypropane. Immunocytochemistry was carried out by using antibodies raised against purified 35-kDa Cys-EP or against mitochondrial malate dehydrogenase (each diluted 1:500) (22).
Light Microscopy, Immunocytochemistry, and Terminal Deoxynucleotidyltransferase-Mediated dUTP Nick End Labeling (TUNEL) Assay.
Endosperm with attached cotyledons was fixed in PBS-buffered formaldehyde [4% formaldehyde (wt/vol)] at 4°C overnight and dehydrated in a graded ethanol series. Ethanol was removed from the tissue by soaking in 100% isopropanol and xylene for 2 h each before infiltrating in Paraplast Plus (Sigma) by using xylene as the carrier. Sections (6–8 μm) were cut with a steel knife, transferred to 3-aminopropyltriethoxysilane (TESPA; Sigma)-coated slides and incubated on a drop of water at 45°C until the water was completely evaporated. Sections were deparaffinized by washing in xylene for 15 min and rehydrated in an ethanol series.
For immunocytochemistry, sections were rinsed with PBS for 15 min, permeabilized in 5% Tween-20 in PBS for up to 2 h at room temperature, and rinsed in PBS to remove the detergent. Nonspecific sites were blocked with 2% BSA in PBS containing 0.02% sodium azide and 0.05% Tween-20 for 1 h at room temperature. The primary antibodies were diluted in blocking solution (anti-Cys-EP 1:500 and anti-propeptide 1:100) and applied overnight at 4°C. Preimmune serum was used for the negative control. Sections were washed in PBS containing 0.05% Tween-20 and incubated with an affinity-purified FITC-labeled goat anti-rabbit secondary antibody (Rockland, Gilbertsville, PA) diluted in blocking solution (working concentration 2 μg/ml) for 3 h at room temperature in the dark. After a final wash in PBS, sections were mounted in fluorescence medium [100 mM Tris, pH 8.0/45% (vol/vol) glycerol/18 mM 1,4-diazabicyclo-(2.2.2)octane] and examined in a Zeiss Axioskop epifluorescence microscope.
For the TUNEL assay, sections were prepared as described above, but permeabilized with 20 μg/ml of proteinase K in 10 mM Tris⋅HCl, pH 7.5 for 30 min at 30°C. Nick end-labeling was performed at 37°C for 1 h according to the manufacturer’s instructions (Boehringer Mannheim), but FITC-labeled nucleotides were diluted (1:3) in terminal transferase buffer (30 mM Tris, pH 7.2/140 mM sodium cacodylate/1 mM CoCl2) to minimize unspecific labeling. Terminal transferase was omitted for the negative control. Nuclear DNA was counterstained with 0.1 μg/ml of 4,6-diamidino-2-phenylindole (DAPI; Sigma) for 5 min at room temperature, washed with PBS, and mounted in fluorescence medium.
Results
Endosperm Morphology in Germinating Castor Bean.
Castor beans store oil and proteins in a living endosperm that surrounds the cotyledons. In germinating seedlings, the radicula emerges on day 2 (Fig. 1a), and the seed coat is cast off on day 3 (Fig. 1f). On day 4, the growing cotyledons separate the endosperm into two halves (Fig. 1l). By day 5, the endosperm is soft and can be removed from the cotyledons because a slimy layer of collapsed cells has developed between the two tissues (Fig. 1r). On day 6, when the storage reserves are depleted, the endosperm becomes increasingly slimy and detaches. The changes in morphology during storage mobilization can be followed under the light microscope by differential interference contrast (DIC) (Fig. 1 b, g, m, and s). At day 2 the endosperm cells are still filled with oil and protein bodies (Fig. 1b), which gradually disappear, and the cells develop a large central vacuole surrounded by a thin layer of cytoplasm (Fig. 1s). The slimy layer of collapsed endosperm cells near the cotyledons becomes visible at day 3 (Fig. 1g) and increases at day 4 (Fig. 1m) and day 5 (Fig. 1s).
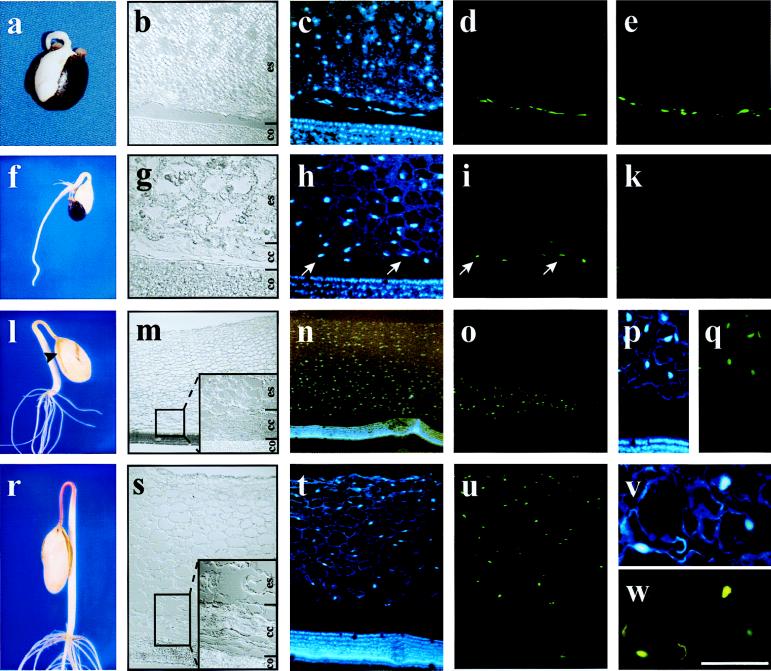
Figure 1.
Nuclear DNA fragmentation in germinating Ricinus endosperm. Sections were prepared from endosperm of 2-day-old (a–e), 3-day-old (f–k), 4-day-old (l–q), and 5-day-old (r–w) seedlings and visualized by using DIC optics (b, g, m, and s; co, cotyledons; cc, collapsed cells; es, living endosperm). Nuclei were stained with DAPI (c, h, n, p, t, and v), and DNA fragmentation was detected by TUNEL (d, i, o, q, u, and w). (e and k) Negative controls of the TUNEL assay in sections from 2-day-old (e) and 3-day-old (k) endosperm. (a–e) Two-day-old seedlings (b) seen by using DIC optics, (c) stained with DAPI, and (d) showing TUNEL-positive cells close to the cotyledons; (e) negative control for TUNEL assay also shows labeling close to the cotyledon. (f–k) Three-day-old seedling (g) seen by using DIC optics, (h) stained with DAPI, and (i) showing TUNEL-positive cells in the first two cell layers next to the cotyledons; (k) negative control for TUNEL assay shows no labeling on endosperm cells. (l–q) Four-day-old seedling (m) seen by using DIC optics showing the whole endosperm with attached cotyledons, (n) stained with DAPI, (o) showing TUNEL-positive nuclei with no labeling on the cotyledons, (p) higher magnification of a section stained with DAPI, and (q) same section as p showing TUNEL-positive cells exclusively in the endosperm. (r–w) Five-day-old seedling (s) section of whole endosperm with attached cotyledons, (t) stained with DAPI, (u) showing TUNEL-positive cells in the whole endosperm with no labeling on the cotyledons, (v) higher magnification of nuclei stained with DAPI, and (w) same section as v shows TUNEL-positive cells. [Scale bar: (a and f) 2 cm; (l and r) 3.5 cm; (m–o) 800 μm; (s–u) 400 μm; (b–e, h, i, k, p, and q) 200 μm; (g, v, and w) 75 μm.]
In Situ Detection of Nuclear DNA Fragmentation by TUNEL in Germinating Endosperm.
Nuclear DNA fragmentation during PCD can be detected by reagents that react with the exposed 3′ hydroxyls on the nucleosomal units (Fig. 1 d, i, o, q, u, and w). The assay involves end-labeling DNA fragments by using terminal deoxynucleotidyl transferase with dUTP conjugated to a fluorochrome. Counterstaining of nucleic DNA with DAPI demonstrates that nuclei are present in all cells except the collapsed endosperm cells (Fig. 1 c, h, n, p, t, and v). At day 2 no nuclear DNA fragmentation was detected; the labeling near the cotyledons was the result of unspecific incorporation of labeled nucleotides, because it also was found in the negative control (Fig. 1 d and e). On day 3 the first signs of DNA degradation were detected in the endosperm cell layers next to the cotyledons (Fig. 1i). The arrows indicate corresponding cells stained with DAPI (Fig. 1h). No signal was detectable in the negative control (Fig. 1k). By day 4 approximately 1/3 of the endosperm cells exhibited nuclear DNA fragmentation (Fig. 1 o and q), compared with DAPI staining (Fig. 1 n and p), and there was no TUNEL labeling in the negative control (not shown). At day 5 nuclear DNA fragmentation could be detected in all endosperm cells (Fig. 1 t–w). Nuclear DNA was not detected by DAPI staining or TUNEL assay in the collapsed endosperm cells near the cotyledons at day 4 (Fig. 1 m–o) or day 5 (Fig. 1 s–u).
The Development of Ricinosomes Is Concomitant with the Progression of Nuclear DNA Fragmentation in Germinating Endosperm.
Ricinosomes were quantified in the endosperm from days 2 to 6, with mitochondria as a control (Table 1), using EM after labeling with antibodies against Cys-EP or mitochondrial malate dehydrogenase. Ricinosomes were difficult to distinguish from other organelles, unless they were labeled (Fig. 2). The distribution of ricinosomes also was followed by immunofluorescence microscopy after labeling with anti-Cys-EP antibody at day 3 (Fig. 3 a and b) and day 5 (Fig. 3 f and g).
Table 1.
Quantification of riconosomes and mitochondria per cell in the outer, middle, and inner layers of germinating castor bean endosperm
| Days of germination | |||||
|---|---|---|---|---|---|
| Ricinosomes | 2 | 3 | 4 | 5 | 6 |
| Outer | 0 | 3.7 ± 2.0 | 16.3 ± 2.6 | 12.5 ± 3.7 | 0 |
| Middle | 0 | 0 | 11.9 ± 2.3 | 3.3 ± 2.5 | — |
| Inner | 0.4 ± 0.5 | 11.7 ± 3.2 | 1.2 ± 1.7 | 0 | — |
| Collapsed cells | 0 | 0 | 0 | — | — |
| Mitochondria | 2 | 3 | 4 | 5 | 6 |
| Outer | 24.5 ± 4.7 | 92.7 ± 18.2 | 74.0 ± 12.8 | 70.3 ± 10.8 | 0 |
| Middle | 14.7 ± 2.8 | 86.8 ± 14.2 | 75.9 ± 10.1 | 61.4 ± 10.1 | — |
| Inner | 14.1 ± 2.1 | 73.3 ± 19.4 | 0.6 ± 1.1 | 0 | — |
| Collapsed cells | 0 | 0 | 0 | — | — |
Average and SE were calculated by counting organelles over 7–10 cells for each developmental stage.
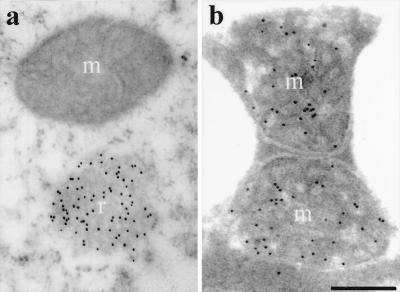
Figure 2.
EM identification of ricinosomes (r) and mitochondria (m) in 5-day-old germinating Ricinus endosperm. Sections were labeled with antibodies against Cys-EP (a) or mitochondrial malate dehydrogenase (b). (Scale bar: 0.25 μm.)
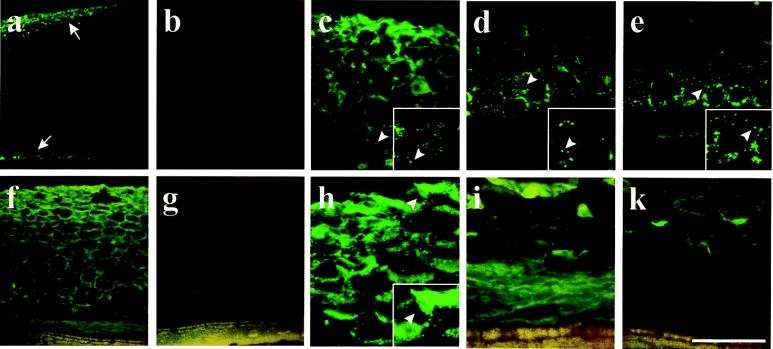
Figure 3.
Development of ricinosomes and cellular distribution of cysteine endopeptidase in germinating Ricinus endosperm. Sections from 3-day-old (a–e) and 5-day-old (f–k) germinating endosperm were labeled with α-Cys-EP antibody (a, c, d, f, h, and i), the preimmune serum (b and g), and the α-propeptide antibody (e and k) by using a FITC-labeled secondary antibody. (a) Cys-EP is detected (arrows) in the outer part of the endosperm and close to the cotyledons in 3-day-old seedlings. (b) Preimmune serum. Higher magnification of the outer part (c) and the inner part (d) of the endosperm shows a punctate labeling in ricinosomes. (e) The α-propeptide antibody labels ricinosomes in the inner part of the endosperm in a punctate pattern. (f) Detection of Cys-EP in the endosperm of 5-day-old seedlings. (g) Preimmune serum. (h) Higher magnification of the outer part of the endosperm shows a mostly diffuse labeling with only a few ricinosomes. (i) Higher magnification of the inner part of the endosperm shows diffuse labeling over the collapsed cells. (k) No labeling is observed over the collapsed cells with the α-propeptide antibody. Arrows in a indicate labeling in the outer and the inner part of 3-day-old endosperm. Arrowheads in c–e and h indicate single ricinosomes. (Insets, c–e and h) A further 2-fold magnification of the region around the arrowheads. [Scale bar: (a and b) 800 μm; (f and g) 400 μm; (k) 200 μm; (c–e, h, and i) 100 μm.]
Ricinosomes were rare at day 2 with only 0.5 ricinosome profile per cell section (4–5 ricinosomes in 10 cells) in the innermost layer of the endosperm closest to the cotyledons. A dramatic increase of ricinosomes took place at day 3 with 11.7 ricinosomes per cell section in the inner layer of the endosperm, when the fragmentation of the nuclear DNA began (Fig. 1i). Ricinosomes were detected in the outer layer of the endosperm at day 3 (Table 1 and Fig. 3a) but not in the middle layer. In the outermost cells of the endosperm, there are fewer protein bodies to be mobilized, and as a consequence, degradation of the cellular material may start earlier. At day 4, ricinosomes are found predominantly in the middle and outer layers. At day 5, ricinosomes were most abundant in the outer layer of the endosperm (Table 1 and Fig. 3f). No fluorescence labeling was obtained by using preimmune serum instead of anti-Cys-EP antibody (Fig. 3 b and g). Accumulation of ricinosomes progressed transversely through the endosperm, similar to the pattern of nuclear DNA fragmentation, with the majority of ricinosomes always next to the collapsed cells. Mitochondria profiles, in contrast, were equally frequent throughout all layers of the endosperm. Their number per cell section increased until day 3, when mobilization of storage reserves was fully active and disappeared during cell collapse (Table 1).
Ricinosomes Accumulate the 45-kDa Proform of Cys-EP, Whereas Disintegration of Ricinosomes During Cell Collapse Releases the 35-kDa Mature Form.
At day 3, ricinosomes were detected in the inner and outer layers of the endosperm by labeling with the antibody against the mature 35-kDa form of Cys-EP (Fig. 3a). Higher magnification (Fig. 3 c and d) revealed a punctate pattern indicating intact ricinosomes. Decoration with antibody directed specifically against the propeptide (Fig. 3e) showed that ricinosomes in the inner layer contained the 45-kDa proform of Cys-EP. By day 5, the situation changed dramatically. Reaction with antibody against the 35-kDa mature form of Cys-EP revealed labeling all over the endosperm, with a strong punctate signal in the outer layers (Fig. 3h), whereas the tissue in the middle had started to disintegrate and punctate label was difficult to see, and there was a diffuse signal in the collapsed cells near the cotyledons (Fig. 3f). This finding parallels the quantification of ricinosomes at day 5 under EM (Table 1). The intense diffuse labeling may be the result of disrupted ricinosomes that have released their Cys-EP (see Western blot below). The labeling in collapsed cells was diffuse (Fig. 3i), and labeling with antibody against the propeptide (Fig. 3k) showed that the Cys-EP is the 35-kDa form with the propeptide cleaved off, whereas the ricinosomes in intact cells contain the 45-kDa proform.
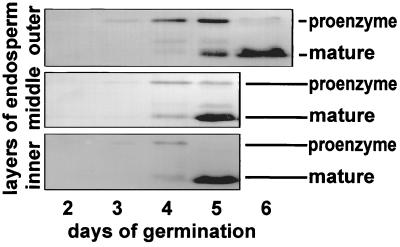
These findings were confirmed by Western blot analysis of the different endosperm layers from days 2 to 6 (Fig. 4). At day 3 only the 45-kDa proform was detected, but by day 4 the mature form started to appear in the inner layer. On day 5 only the mature 35-kDa form was found in the collapsed cells of the inner layer, whereas in the middle part most of the proform was converted into the mature form. In the outer layer the proform was present, together with the mature form. At day 6 the endosperm consisted of slimy remnants containing exclusively the mature Cys-EP.
Figure 4.
Western blot analysis of germinating Ricinus endosperm from days 2–6 in protein extracts of the inner collapsed layer, the middle layer, and the outer layer with α-Cys-EP antibody.
Ricinosomes Containing the 45-kDa Cys-EP Are Found in Developing Castor Bean Seeds.
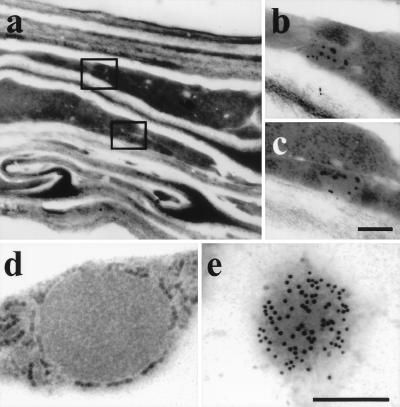
A cDNA clone for a cysteine endoprotease very similar or identical to that one in the germinating endosperm was found in a cDNA library prepared from stages III through V of developing castor beans (22). Protein was extracted from developing seeds at stages I–V. Cys-EP was detected by Western blot analysis. The 45-kDa proform of Cys-EP was found only in the seed, and not in the pericarp, in the latest stage of seed development (data not shown). Immuno-EM identified ricinosomes in a layer of collapsing cells at the outer edge of the seed (Fig. 5 a–c). They might represent either the senescing nucellus, which surrounds the embryo sack and collapses as soon as seed maturation is completed, or an integument developing into the seed coat.
Figure 5.
Identification of ricinosomes in developing Ricinus seeds in the collapsed cells near the seed coat (a–c) and daylily petals (d and e) by labeling with α-Cys-EP antibody. (a) Overview of collapsing cells in Ricinus. (b and c) Immunocytochemistry. (d) Ricinosome in Hemerocallis petal. (e) Immunocytochemistry. [Scale bar: (a) 1 cm; (b and c) 0.75 μm; and (d and e) 0.25 μm.]
Ricinosome-Like Organelles Containing a 45-kDa Cys-EP Are Found in Hemerocallis Petals.
The papain-type KDEL-protease is a marker enzyme for ricinosomes (22), and because a homologous cysteine endoprotease exists in daylily (12), we looked for ricinosomes in petals 8 h after flowering. Ricinosome-like organelles with ribosome-like particles on the membrane in the cytoplasm surrounding the large central vacuole (Fig. 5d) were labeled with antibody directed against the Cys-EP from Ricinus (Fig. 5e). Western blot analysis revealed the 45-kDa proform of Cys-EP in petals, but not in the ovary (data not shown).
Discussion
PCD in Senescing Ricinus Endosperm.
Many plant tissues exhibit a genetic program leading to cell death at different stages of development, where the production of specific proteases is followed by a loss of membrane permeability and a decrease in protein content. In Ricinus endosperm, PCD begins in the cell layers closest to the cotyledon and progresses transversely across to the opposite side of the tissue over 3–4 days. This process was shown by TUNEL-positive nuclei, indicating the presence of chromosomal DNA breakage. The level of dUTP was 1/4 of the concentration recommended for animal cells to prevent end-labeling artifacts (24), and the absence of TUNEL-positive cotyledon nuclei served as an internal negative control. Plasma membrane integrity was maintained during endosperm senescence, as judged by EM, but membrane blebbing, chromatin condensation, or the formation of apoptotic bodies was not observed. It is accepted that the presence of TUNEL-positive nuclei is an insufficient criterion for defining PCD as apoptosis (25). In barley aleurone cells, PCD is hormonally regulated by gibberellic acid, but does not resemble apoptosis; instead cell death is highly correlated with the extent of vacuolation (26).
Ricinosomes and PCD.
The detection of ricinosomes is concomitant with the appearance of TUNEL-positive nuclei in senescing endosperm and parallels their progression across the tissue. As long as the ricinosomes are intact, the Cys-EP is in the relatively inactive 45-kDa proform, which has enzymatic activity, but is approximately 50–100 times less active than the mature form. Loss of ricinosome integrity may result from a change in the permeability of the tonoplast, leading to acidification of the cytoplasm. The low pH could trigger autocatalytic cleavage of the Cys-EP propeptide, as for papain (27) and other cysteine endoproteases.
The signal initiating PCD in Ricinus endosperm is not known. One mechanism in animals involves leakage of cytochrome c from mitochondria, regulated by BCL-2 and BAX. Cytochrome c binds to the CED-4 receptor molecule in the outer mitochondrial membrane. This complex recruits procaspase 9, which undergoes autocatalytic cleavage to form the active enzyme, which initiates the caspase cascade (28). The possibility of this regulation also occurring in plants is suggested by the discovery of plant CED-4 homologues, known as apoptotic ATPases, in tobacco and Arabidopsis (29).
The involvement of a caspase in plant PCD has been indicated in the hypersensitive response of tobacco leaves to Pseudomonas syringae, based on the inhibition of PCD by caspase-specific inhibitors (30). The same technique indicated no involvement of caspases in PCD in suspension-cultured soybean cells induced by P. syringae or oxidative stress, whereas ectopic expression of cystatin, a specific cysteine endoprotease inhibitor, blocked PCD (11). Cysteine endoproteases are implicated in PCD in many senescing plant tissues, but only some have a C-terminal KDEL motif. They accumulate later than the cysteine endoproteases involved in mobilization of storage proteins. Whether cysteine endoproteases are activated as part of a caspase cascade, or by another mechanism, they are an essential part of plant PCD.
Cysteine Endoproteases with a C-Terminal KDEL Are Marker Enzymes for Ricinosomes.
Papain-type cysteine endoproteases with a C-terminal KDEL motif have been reported in many plant species, often as cDNAs from senescing tissue, such as Hemerocallis petals (12), Phalaenopsis (13), and pea ovaries (15). The 41-kDa precursor of proteinase A in V. sativa (Z38495) was detectable from day 3 after imbibition, but the mature 29-kDa form first appeared in the cotyledons at day 6 after imbibition (17), similar to the Ricinus Cys-EP (22). We now have shown that senescing Hemerocallis petals contain a ricinosome-like organelle that is labeled by the antibody to Ricinus Cys-EP. These results raise the possibility that ricinosomes are not unique to Ricinus and may be found in other plant species, particularly those in which papain-type cysteine endoproteases with a C-terminal KDEL motif have been reported.
ER-Derived Structures in Plants.
Several membrane-bound structures originate directly from the ER. Coat protein II-coated vesicles, which transport proteins from the ER to the cis-Golgi, bud off from the ER (31), and a similar process gives rise to oil bodies (32). In tropical cereals such as maize (33), the prolamin storage proteins accumulate and form protein bodies within the ER. Ricinosomes also were called dilated cisternae, because electron micrographs showed that they were contiguous with the ER and were surrounded by a ribosome-coated membrane (20, 22). Furthermore, the ER-resident proteins binding protein (BiP), calreticulin, calnexin, and protein disulfide isomerase are found in purified ricinosomes (M.S. and C.G., unpublished observations). Because the C-terminal KDEL motif targets proteins by acting as an ER retrieval signal by recycling from the Golgi (31), it is interesting that the Cys-EP is targeted to an organelle that derives from the ER and eventually separates from it. In maturing Ricinus seeds, precursor accumulating (PAC) vesicles have been suggested to transport proteins directly from the ER to the vacuole (34). These vesicles contain entrapped BiP, but the presence of Golgi-derived glycoproteins indicates that PAC vesicles also may fuse with trans-Golgi vesicles. Ricinosomes are different from PAC vesicles, with at least twice their diameter, and appear during senescence, whereas PAC vesicles occur in developing cotyledons and endosperm during storage protein accumulation. The structural organization of the ER, in particular the definition of ER subdomains for the biogenesis of transport vesicles, protein bodies, oil bodies, or ricinosomes will need increasing attention.
Acknowledgments
We thank Dr. Frantisek Kalousek for the preparation of antibodies against the purified CysEP. This work was supported by the Deutsche Forschungsgemeinschaft (Gi 154/19-2).
Abbreviations
- Cys-EP
castor bean cysteine endopeptidase
- DAPI
4,6-diamidino-2-phenylindole
- DIC
differential interference contrast
- EM
electron microscopy
- ER
endoplasmic reticulum
- PCD
programmed cell death
- TUNEL
terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling
References
- 1.Ellis R E, Yuan J Y, Horvitz H R. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- 2.Steller H. Science. 1995;267:1445–1499. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- 3.Kerr J F R, Wyllie A H, Currie A R. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenberg J T. Proc Natl Acad Sci USA. 1996;93:12094–12097. doi: 10.1073/pnas.93.22.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pennel R I, Lamb C. Plant Cell. 1997;9:1157–1168. doi: 10.1105/tpc.9.7.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green D G. Cell. 1998;94:695–698. doi: 10.1016/s0092-8674(00)81728-6. [DOI] [PubMed] [Google Scholar]
- 7.Nicholson D W, Ali A, Thornberry N A, Vaillancourt J P, Ding C K, Gallant M, Gareau Y, Griffin P R, Labelle M, Labeznik Y A. Nature (London) 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 8.Cohen G M. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minami A, Fukuda H. Plant Cell Physiol. 1995;36:1599–1606. [PubMed] [Google Scholar]
- 10.Ye Z H, Varner J E. Plant Mol Biol. 1996;30:1233–1246. doi: 10.1007/BF00019555. [DOI] [PubMed] [Google Scholar]
- 11.Solomon M, Belenghi B, Delledonne M, Menachem E, Levine A. Plant Cell. 1999;11:431–443. doi: 10.1105/tpc.11.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valpuesta V, Lange N E, Guerrero C, Reid M S. Plant Mol Biol. 1995;28:575–582. doi: 10.1007/BF00020403. [DOI] [PubMed] [Google Scholar]
- 13.Nadeau J A, Zhang X S, Li J, O’Neill S D. Plant Cell. 1996;8:213–239. doi: 10.1105/tpc.8.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka T, Minamikawa T, Yamauchi D, Ogushi Y. Plant Physiol. 1993;101:421–428. doi: 10.1104/pp.101.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cercos M, Santamaria S, Carbonell J. Plant Physiol. 1999;119:1341–1348. doi: 10.1104/pp.119.4.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuo A, Cappelluti S, Cervantes-Cervantes M, Rodriguez M, Bush D S. Plant Cell. 1996;8:259–269. doi: 10.1105/tpc.8.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becker C, Senyuk V I, Shutov A D, Nong V H, Fischer J, Horstmann C, Müntz K. Eur J Biochem. 1997;248:304–312. doi: 10.1111/j.1432-1033.1997.00304.x. [DOI] [PubMed] [Google Scholar]
- 18.Akasofu H, Yamauchi D, Mitsuhashi W, Minamikawa T. Nucleic Acids Res. 1989;17:6733. doi: 10.1093/nar/17.16.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mollenhauer H H, Totten C. Plant Physiol. 1970;46:794–799. doi: 10.1104/pp.46.6.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vigil E L. J Cell Biol. 1970;46:435–454. doi: 10.1083/jcb.46.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gietl C, Wimmer B, Adamec J, Kalousek F. Plant Physiol. 1997;113:863–871. doi: 10.1104/pp.113.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmid M, Simpson D, Kalousek F, Gietl C. Planta. 1998;206:466–475. doi: 10.1007/s004250050423. [DOI] [PubMed] [Google Scholar]
- 23.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.Wang H, Li J, Bostock R M, Gilchrist D G. Plant Cell. 1996;8:375–391. doi: 10.1105/tpc.8.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones A M, Dangl J L. Trends Plant Sci. 1996;1:114–119. [Google Scholar]
- 26.Bethke P C, Lonsdale J E, Fath A, Jones R L. Plant Cell. 1999;11:1033–1045. doi: 10.1105/tpc.11.6.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vernet T, Berti P J, de Montigny C, Musil R, Tessier D C, Ménard R, Magny M C, Storer A C, Thomas D Y. J Biol Chem. 1995;270:10838–10846. doi: 10.1074/jbc.270.18.10838. [DOI] [PubMed] [Google Scholar]
- 28.Raff M. Nature (London) 1998;396:119–122. doi: 10.1038/24055. [DOI] [PubMed] [Google Scholar]
- 29.Aravind L, Dixit V M, Koonin E V. Trends Biochem Sci. 1999;24:47–53. doi: 10.1016/s0968-0004(98)01341-3. [DOI] [PubMed] [Google Scholar]
- 30.Del Pozo O, Lam E. Curr Biol. 1998;8:1129–1132. doi: 10.1016/s0960-9822(98)70469-5. [DOI] [PubMed] [Google Scholar]
- 31.Møgelsvang S, Simpson D J. J Plant Physiol. 1998;153:1–15. [Google Scholar]
- 32.Huang A H C. Annu Rev Plant Physiol Mol Biol. 1992;43:177–200. [Google Scholar]
- 33.Lending C R, Larkins B A. Plant Cell. 1989;1:1011–1023. doi: 10.1105/tpc.1.10.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hara-Nishimura I, Shimada T, Hatano K, Takeuchi Y, Nishimura M. Plant Cell. 1998;10:825–836. doi: 10.1105/tpc.10.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]