Abstract
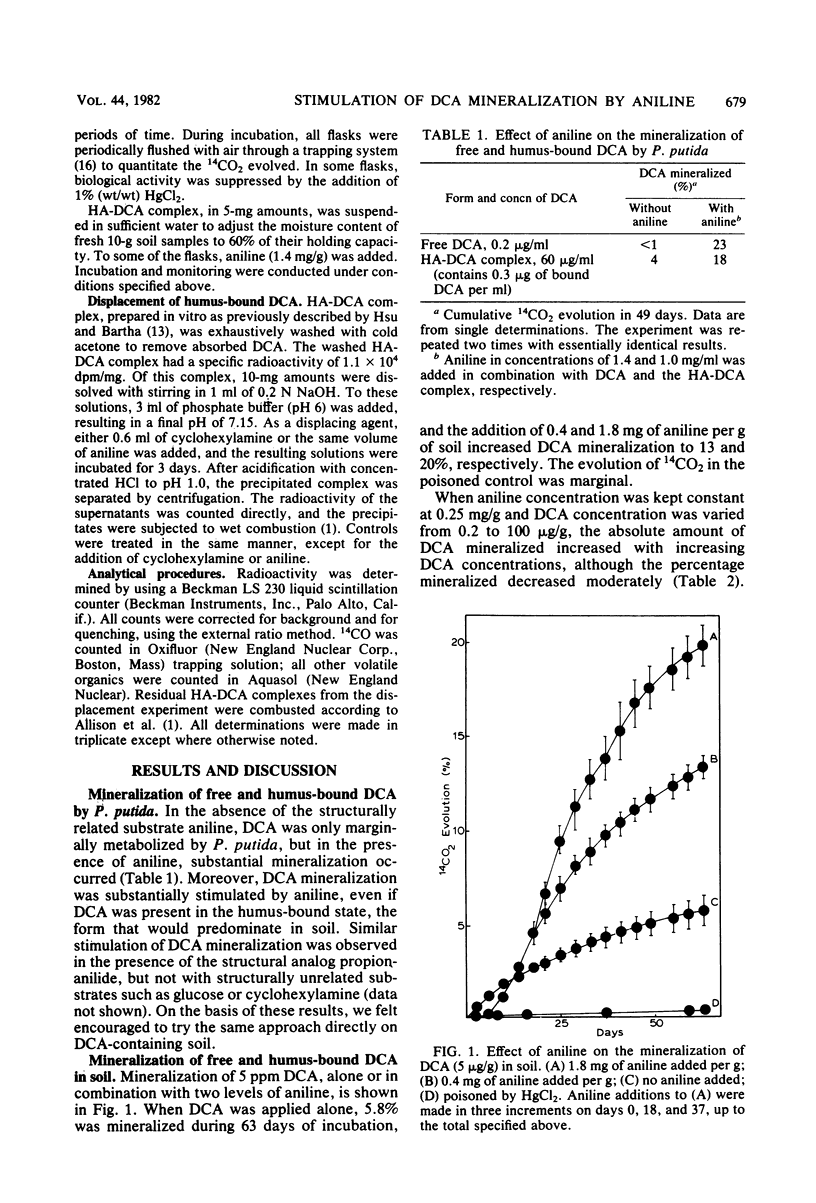
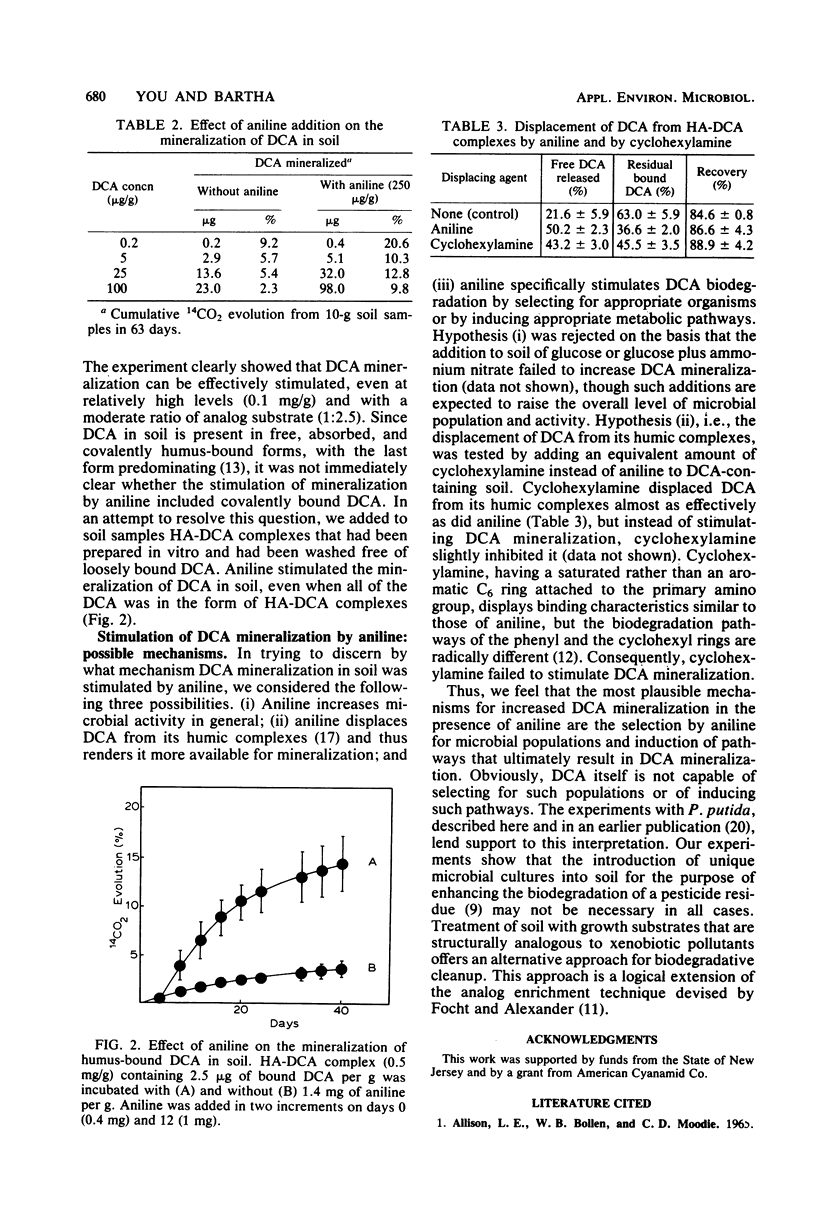
Mineralization of free and of humus-bound 3,4-dichloroaniline (DCA) by a Pseudomonas putida strain isolated by analog enrichment was greatly enhanced in the presence of aniline. The addition of aniline to soil that contained 0.2 to 100 micrograms of DCA per g in free or in humus-bound form increased the mineralization rates of DCA severalfold. Within the concentration ranges tested, absolute mineralization of DCA per unit time was positively correlated with both increasing DCA and increasing aniline concentrations. The specific enrichment of microbial populations and the induction of pathways that can co-metabolize DCA are the most plausible explanations for the effect of aniline. The observed phenomenon points to a potential approach for eliminating xenobiotic pollutants from contaminated soils.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartha R. Fate of herbicide-derived chloroanilines in soil. J Agric Food Chem. 1971 Mar-Apr;19(2):385–387. doi: 10.1021/jf60174a024. [DOI] [PubMed] [Google Scholar]
- Bartha R., Linke H. A., Pramer D. Pesticide transformations: production of chloroazobenzenes from chloroanilines. Science. 1968 Aug 9;161(3841):582–583. doi: 10.1126/science.161.3841.582. [DOI] [PubMed] [Google Scholar]
- Bordeleau L. M., Rosen J. D., Bartha R. Herbicide-derived chloroazobenzene residues: pathway of formation. J Agric Food Chem. 1972 May-Jun;20(3):573–578. doi: 10.1021/jf60181a001. [DOI] [PubMed] [Google Scholar]
- Chisaka H., Kearney P. C. Metabolism of propanil in soils. J Agric Food Chem. 1970 Sep-Oct;18(5):854–858. doi: 10.1021/jf60171a032. [DOI] [PubMed] [Google Scholar]
- Daughton C. G., Hsieh D. P. Accelerated parathion degradation in soil by inoculation with parathion-utilizing bacteria. Bull Environ Contam Toxicol. 1977 Jul;18(1):48–56. doi: 10.1007/BF01686304. [DOI] [PubMed] [Google Scholar]
- Focht D. D., Alexander M. Bacterial degradation of diphenylmethane, a DDT model substrate. Appl Microbiol. 1970 Oct;20(4):608–611. doi: 10.1128/am.20.4.608-611.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu T. S., Bartha R. Hydrolyzable and nonhydrolyzable 3,4-dichloroaniline-humus complexes and their respective rates of biodegradation. J Agric Food Chem. 1976 Jan-Feb;24(1):118–122. doi: 10.1021/jf60203a021. [DOI] [PubMed] [Google Scholar]
- Kanner D., Bartha R. Growth of Nocardia rhodochrous on acetylene gas. J Bacteriol. 1979 Jul;139(1):225–230. doi: 10.1128/jb.139.1.225-230.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinucci A. C., Bartha R. Apparatus for monitoring the mineralization of volatile C-labeled compounds. Appl Environ Microbiol. 1979 Nov;38(5):1020–1022. doi: 10.1128/aem.38.5.1020-1022.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Still C. C., Hsu T. S., Bartha R. Soil-bound 3,4-dichloroaniline: source of contamination in rice grain. Bull Environ Contam Toxicol. 1980 Apr;24(4):550–554. doi: 10.1007/BF01608154. [DOI] [PubMed] [Google Scholar]


