Abstract
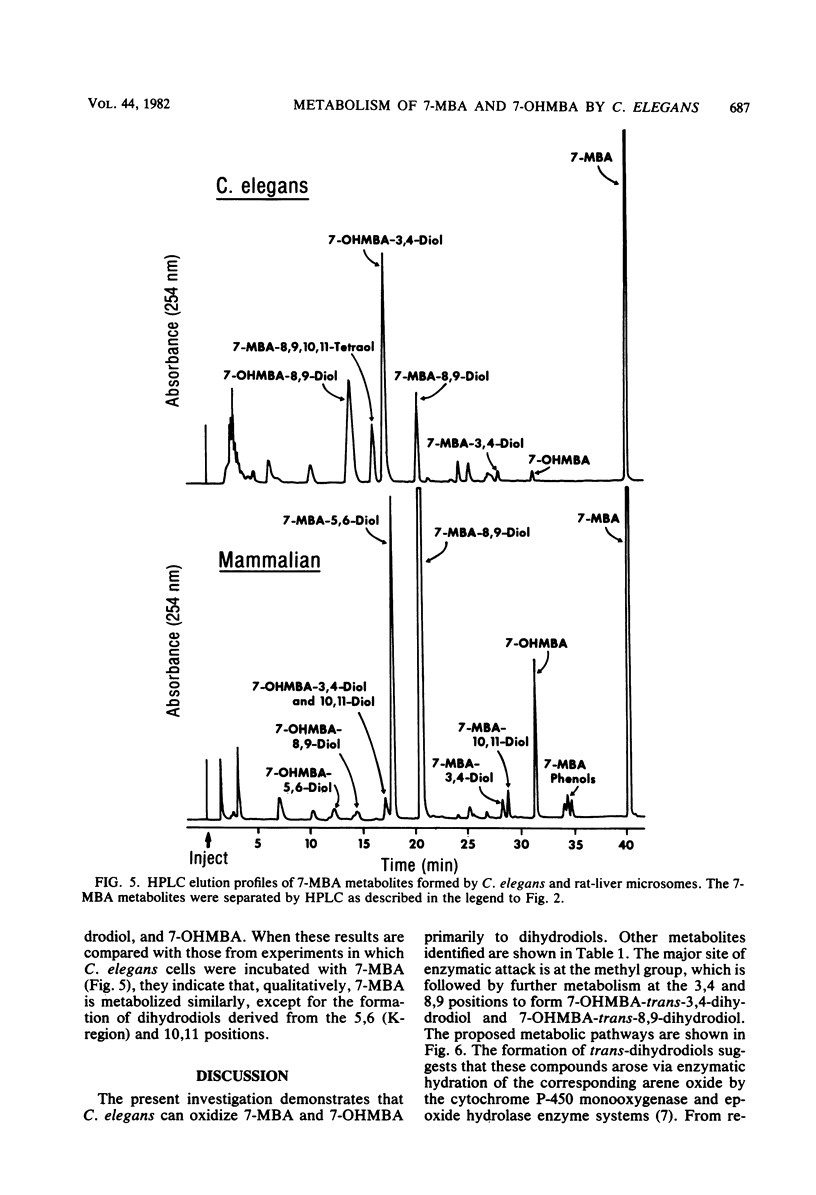
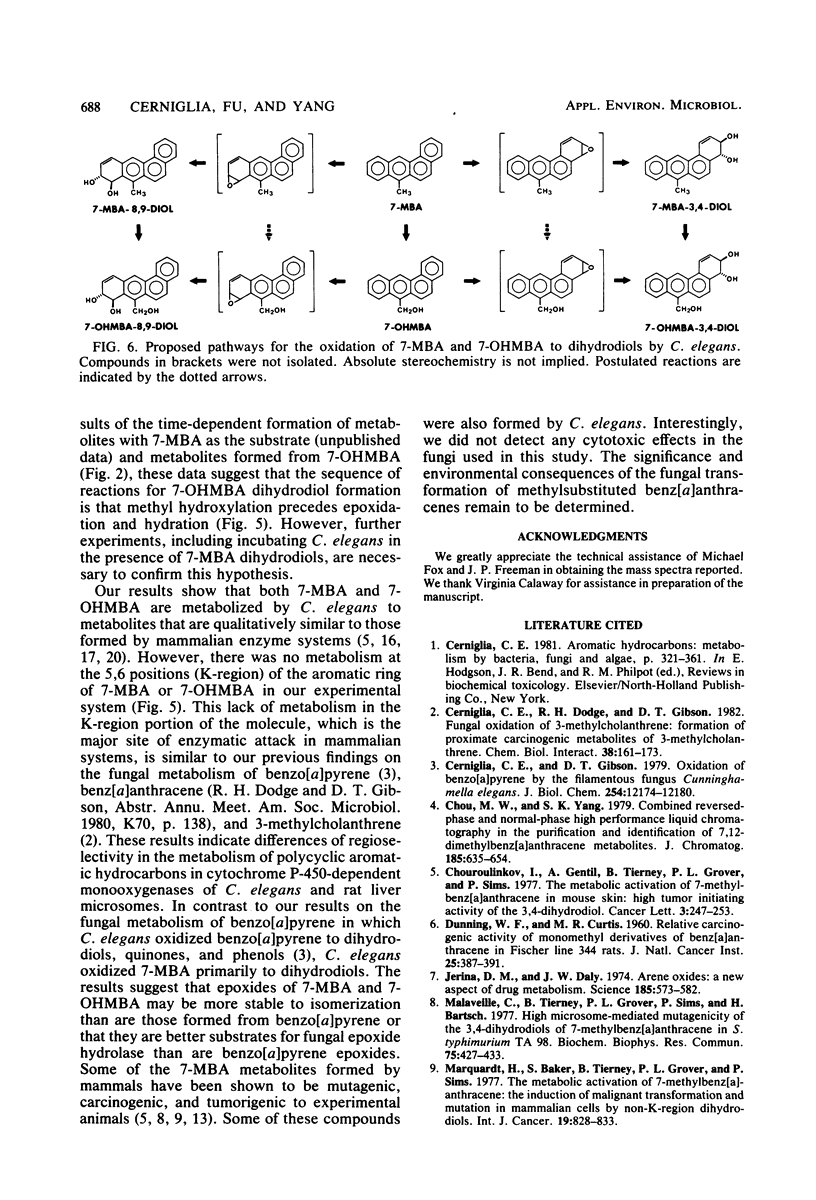
The fungal metabolism of 7-methylbenz[a]anthracene (7-MBA) and 7-hydroxymethylbenz[a]anthracene (7-OHMBA) was studied. 7-MBA was metabolized by Cunninghamella elegans to form 7-OHMBA-trans-8,9-dihydrodiol and 7-OHMBA-trans-3,4-dihydrodiol as the predominant metabolites. Other metabolites were identified as 7-OHMBA, 7-MBA-trans-8,9-dihydrodiol and 7-MBA-trans-3,4-dihydrodiol, and 7-MBA-8,9,10,11-tetraol. Incubation of 7-OHMBA with C. elegans cells indicated that 7-OHMBA-trans-8,9-dihydrodiol and 7-OHMBA-trans-3,4-dihydrodiol were major metabolites. The metabolism of 7-MBA by rat liver microsomes from 3-methylcholanthrene-treated rats showed that the metabolites were qualitatively similar to those formed by C. elegans, except additional dihydrodiol metabolites were formed at the 5,6 and 10,11 positions. The metabolites formed were isolated by high-performance liquid chromatography and identified by comparing their chromatographic, UV-visible absorption and mass spectral properties with those of reference compounds.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cerniglia C. E., Dodge R. H., Gibson D. T. Fungal oxidation of 3-methylcholanthrene: formation of proximate carcinogenic metabolites of 3-methylcholanthrene. Chem Biol Interact. 1982 Jan;38(2):161–173. doi: 10.1016/0009-2797(82)90037-0. [DOI] [PubMed] [Google Scholar]
- Cerniglia C. E., Gibson D. T. Oxidation of benzo[a]pyrene by the filamentous fungus Cunninghamella elegans. J Biol Chem. 1979 Dec 10;254(23):12174–12180. [PubMed] [Google Scholar]
- Chou M. W., Yang S. K. Combined reversed-phase and normal-phase high-performance liquid chromatography in the purification and identification of 7,12-dimethylbenz[a]anthracene metabolites. J Chromatogr. 1979 Dec 20;185:635–654. doi: 10.1016/s0021-9673(00)85637-x. [DOI] [PubMed] [Google Scholar]
- Jerina D. M., Daly J. W. Arene oxides: a new aspect of drug metabolism. Science. 1974 Aug 16;185(4151):573–582. doi: 10.1126/science.185.4151.573. [DOI] [PubMed] [Google Scholar]
- Malaveille C., Tierney B., Grover P. L., Sims P., Bartsch H. High microsome-mediated mutagenicity of the 3,4-dihydrodiol of 7-methylbenz[a]anthracene in S. typhimurium TA 98. Biochem Biophys Res Commun. 1977 Mar 21;75(2):427–433. doi: 10.1016/0006-291x(77)91060-9. [DOI] [PubMed] [Google Scholar]
- Marquardt H., Baker S., Tierney B., Grover P. L., Sims P. The metabolic activation of 7-methylbenz(a)anthracene: the induction of malignant transformation and mutation in mammalian cells by non-K-region dihydrodiols. Int J Cancer. 1977 Jun 15;19(6):828–833. doi: 10.1002/ijc.2910190614. [DOI] [PubMed] [Google Scholar]
- Sims P. Studies on the metabolism of 7-methylbenz-[a]anthracene and 7,12-dimethylbenz[1]anthracene and its hydroxymethyl derivatives in rat liver and adrenal homogenates. Biochem Pharmacol. 1970 Jul;19(7):2261–2275. doi: 10.1016/0006-2952(70)90125-5. [DOI] [PubMed] [Google Scholar]
- Sims P. The metabolism of 7- and 12-methylbenz[a]anthracene and their derivatives. Biochem J. 1967 Nov;105(2):591–598. doi: 10.1042/bj1050591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaga T. J., Gleason G. L., Mills G., Ewald L., Fu P. P., Lee H. M., Harvey R. G. Comparison of the skin tumor-initiating activities of dihydrodiols and diol-epoxides of various polycyclic aromatic hydrocarbons. Cancer Res. 1980 Jun;40(6):1981–1984. [PubMed] [Google Scholar]
- Tierney B., Abercrombie B., Walsh C., Hewer A., Grover P. L., Sims P. The preparation of dihydrodiols from 7-methylbenz[a]-anthracene. Chem Biol Interact. 1978 Jun;21(2-3):289–298. doi: 10.1016/0009-2797(78)90027-3. [DOI] [PubMed] [Google Scholar]
- Tierney B., Hewer A., Walsh C., Grover P. L., Sims P. The metabolic activation of 7-methylbenz(a)anthracene in mouse skin. Chem Biol Interact. 1977 Aug;18(2):179–193. doi: 10.1016/0009-2797(77)90005-9. [DOI] [PubMed] [Google Scholar]
- Vigny P., Duquesne M., Coulomb H., Lacombe C. Metabolic activation of polycyclic hydrocarbons. Fluorescence spectral evidence is consistent with metabolism at the 1,2- and 3,4-double bonds of 7-methylbenz[a]anthracene. FEBS Lett. 1977 Mar 15;75(1):9–12. doi: 10.1016/0014-5793(77)80041-0. [DOI] [PubMed] [Google Scholar]
- Wu J., Wong L. K. Microbial transformations of 7,2-dimethylbenz[a]anthracene. Appl Environ Microbiol. 1981 Mar;41(3):843–845. doi: 10.1128/aem.41.3.843-845.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. K., McCourt D. W., Gelboin H. V., Miller J. R., Roller P. P. Stereochemistry of the hydrolysis products and their acetonides of two stereoisomeric benzo[a]pyrene 7,8-diol 9,10-epoxides. J Am Chem Soc. 1977 Jul 20;99(15):5124–5130. doi: 10.1021/ja00457a036. [DOI] [PubMed] [Google Scholar]



