Abstract
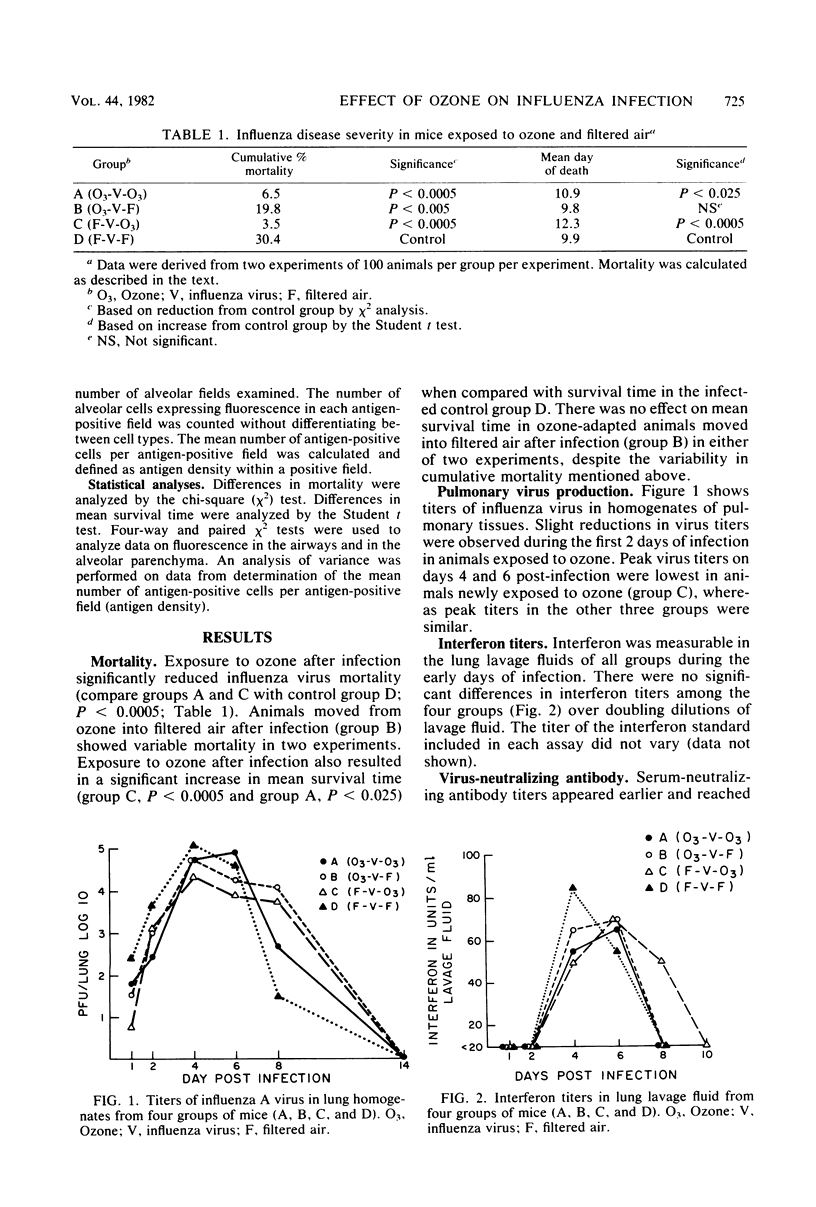
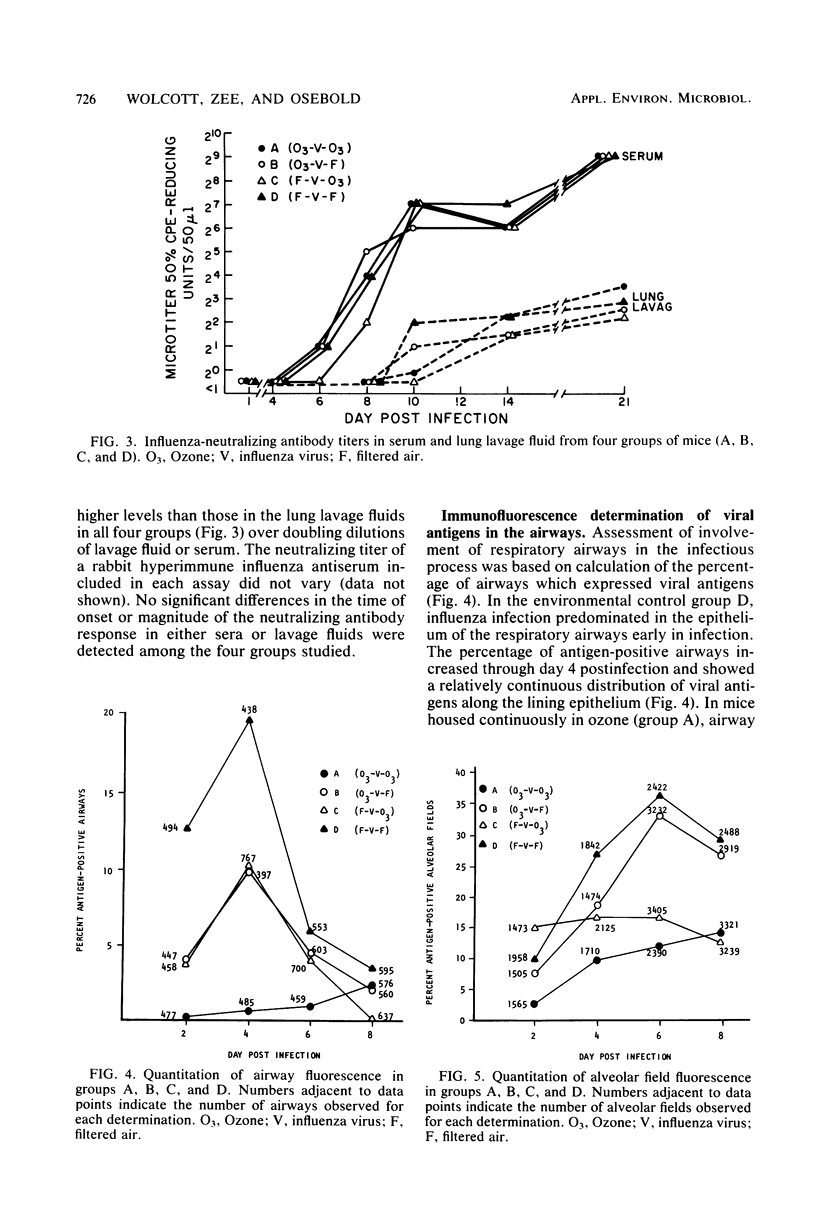
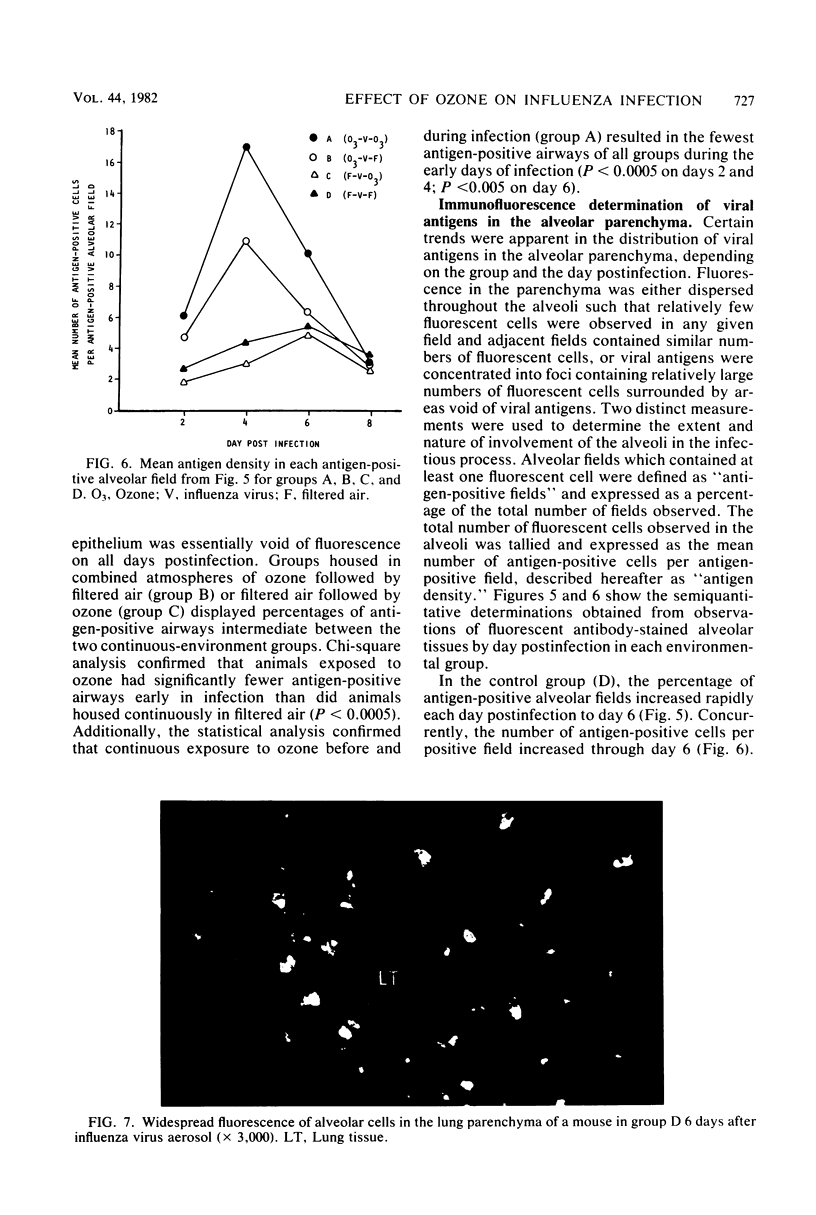
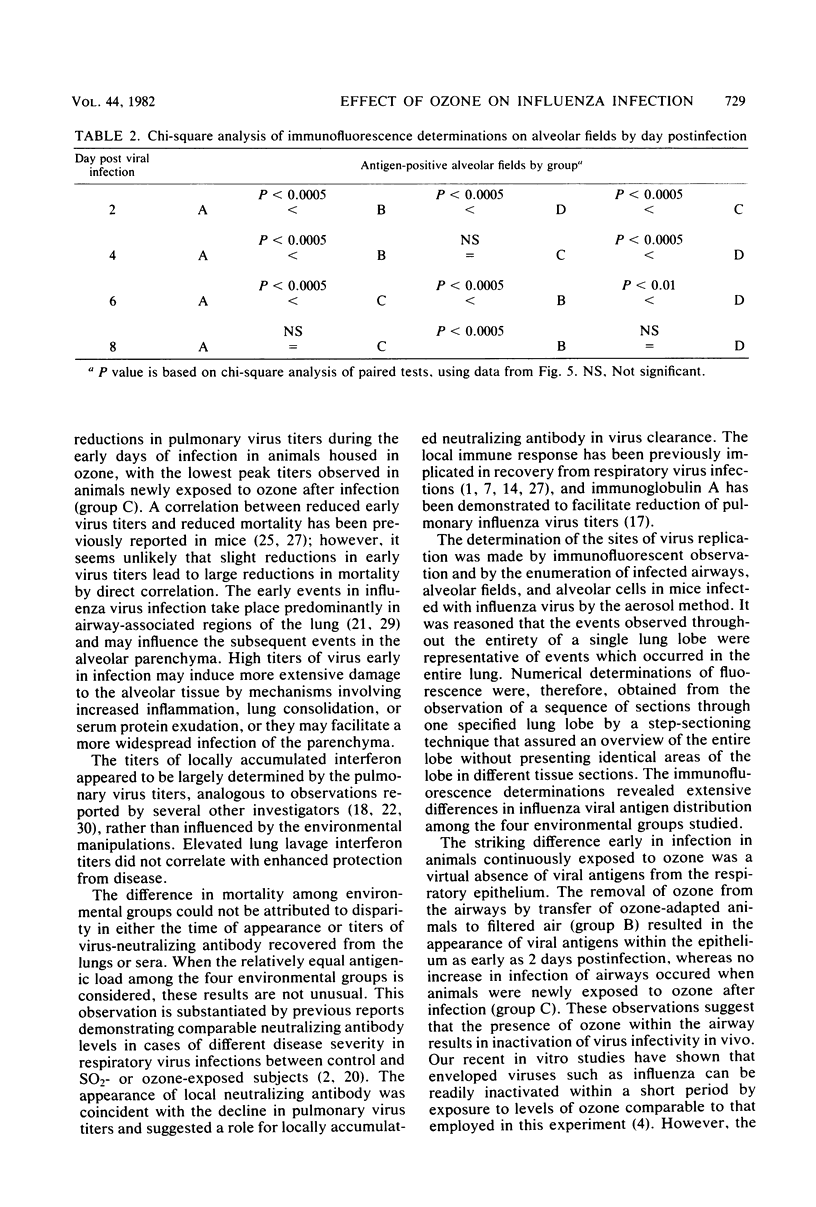
Exposure to ambient levels of ozone (0.5 ppm) was shown to alter the pathogenesis of respiratory infection after aerosol infection of mice with influenza A virus. A semiquantitative method for determination of the sites of virus replication by direct immunofluorescence indicated that exposure to ozone reduced the involvement of respiratory epithelium in the infectious process and resulted in a less widespread infection of the alveolar parenchyma. Furthermore, the ozone-mediated alteration in viral antigen distribution was consistent with significantly reduced influenza disease mortality and prolonged survival time, but only when the oxidant was present during the course of infection. Reduced disease severity in ozone-exposed animals appeared to be independent of peak pulmonary virus titers, pulmonary interferon titers, and pulmonary and serum-neutralizing antibody titers. These studies suggested that the distribution of influenza virus in the murine lung was a key factor in disease severity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alford R. H., Rossen R. D., Butler W. T., Kasel J. A. Neutralizing and hemagglutination-inhibiting activity of nasal secretions following experimental human infection with A2 influenza virus. J Immunol. 1967 Apr;98(4):724–731. [PubMed] [Google Scholar]
- Andersen I., Jensen P. L., Reed S. E., Craig J. W., Proctor D. F., Adams G. K. Induced rhinovirus infection under controlled exposure to sulfur dioxide. Arch Environ Health. 1977 May-Jun;32(3):120–125. doi: 10.1080/00039896.1977.10667267. [DOI] [PubMed] [Google Scholar]
- Boatman E. S., Frank R. Morphologic and ultrastructural changes in the lungs of animals during acute exposure to ozone. Chest. 1974 Apr;65(Suppl):9S–11S. doi: 10.1378/chest.65.4_supplement.9s. [DOI] [PubMed] [Google Scholar]
- Bolton D. C., Zee Y. C., Osebold J. W. The biological effects of ozone on representative members of five groups of animal viruses. Environ Res. 1982 Apr;27(2):476–484. doi: 10.1016/0013-9351(82)90102-5. [DOI] [PubMed] [Google Scholar]
- Buckley R. D., Loosli C. G. Effects of nitrogen dioxide inhalation on germfree mouse lung. Arch Environ Health. 1969 Apr;18(4):588–595. doi: 10.1080/00039896.1969.10665457. [DOI] [PubMed] [Google Scholar]
- CLARK H. F., SHEPARD C. C. A DIALYSIS TECHNIQUE FOR PREPARING FLUORESCENT ANTIBODY. Virology. 1963 Aug;20:642–644. doi: 10.1016/0042-6822(63)90292-7. [DOI] [PubMed] [Google Scholar]
- Castleman W. L., Tyler W. S., Dungworth D. L. Lesions in respiratory bronchioles and conducting airways of monkeys exposed to ambient levels of ozone. Exp Mol Pathol. 1977 Jun;26(3):384–400. doi: 10.1016/0014-4800(77)90041-7. [DOI] [PubMed] [Google Scholar]
- Cate T. R., Rossen R. D., Douglas R. G., Jr, Butler W. T., Couch R. B. The role of nasal secretion and serum antibody in the rhinovirus common cold. Am J Epidemiol. 1966 Sep;84(2):352–363. doi: 10.1093/oxfordjournals.aje.a120648. [DOI] [PubMed] [Google Scholar]
- Choppin P. W. Replication of influenza virus in a continuous cell line: high yield of infective virus from cells inoculated at high multiplicity. Virology. 1969 Sep;39(1):130–134. doi: 10.1016/0042-6822(69)90354-7. [DOI] [PubMed] [Google Scholar]
- DOHAN F. C., EVERTS G. S., SMITH R. Variations in air pollution and the incidence of respiratory disease. J Air Pollut Control Assoc. 1962 Sep;12:418–422. doi: 10.1080/00022470.1962.10468109. [DOI] [PubMed] [Google Scholar]
- Dungworth D. L., Castleman W. L., Chow C. K., Mellick P. W., Mustafa M. G., Tarkington B., Tyler W. S. Effect of ambient levels of ozone on monkeys. Fed Proc. 1975 Jul;34(8):1670–1674. [PubMed] [Google Scholar]
- FAZEKAS DE ST GROTH S., DONNELLEY M., GRAHAM D. M. Studies in experimental immunology of influenza. VIII. Pathotopic adjuvants. Aust J Exp Biol Med Sci. 1951 Sep;29(5):323–327. doi: 10.1038/icb.1951.39. [DOI] [PubMed] [Google Scholar]
- Fairchild G. A. Effects of ozone and sulfur dioxide on virus growth in mice. Arch Environ Health. 1977 Jan-Feb;32(1):28–33. doi: 10.1080/00039896.1977.10667249. [DOI] [PubMed] [Google Scholar]
- Fairchild G. A., Roan J., McCarroll J. Atmospheric pollutants and the pathogenesis of viral respiratory infection. Sulfur dioxide and influenza infection in mice. Arch Environ Health. 1972 Sep;25(3):174–182. doi: 10.1080/00039896.1972.10666157. [DOI] [PubMed] [Google Scholar]
- Freeman G., Stephens R. J., Coffin D. L., Stara J. F. Changes in dogs' lung after long-term exposure to ozone: light and electron microscopy. Arch Environ Health. 1973 Apr;26(4):209–216. doi: 10.1080/00039896.1973.10666258. [DOI] [PubMed] [Google Scholar]
- French J. G., Lowrimore G., Nelson W. C., Finklea J. F., English T., Hertz M. The effect of sulfur dioxide and suspended sulfates on acute respiratory disease. Arch Environ Health. 1973 Sep;27(3):129–133. doi: 10.1080/00039896.1973.10666340. [DOI] [PubMed] [Google Scholar]
- Iwasaki T., Nozima T. Defense mechanisms against primary influenza virus infection in mice. I. The roles of interferon and neutralizing antibodies and thymus dependence of interferon and antibody production. J Immunol. 1977 Jan;118(1):256–263. [PubMed] [Google Scholar]
- Jao R. L., Wheelock E. F., Jackson G. G. Production of interferon in volunteers infected with Asian influenza. J Infect Dis. 1970 Apr;121(4):419–426. doi: 10.1093/infdis/121.4.419. [DOI] [PubMed] [Google Scholar]
- Kurimura T., Hirano A., Suzuki N., Okuno Y. Influenza virus infection in mice: the effect of avirulent virus infection on the pathogenicity of highly virulent virus. Biken J. 1971 Mar;14(1):65–73. [PubMed] [Google Scholar]
- Loosli C. G., Hertweck M. S., Hockwald R. S. Airborne influenza PR8-A virus infections in actively immunized mice. Arch Environ Health. 1970 Sep;21(3):332–346. doi: 10.1080/00039896.1970.10667248. [DOI] [PubMed] [Google Scholar]
- McLaren C., Potter C. W. The relationship between interferon and virus virulence in influenza virus infections of the mouse. J Med Microbiol. 1973 Feb;6(1):21–32. doi: 10.1099/00222615-6-1-21. [DOI] [PubMed] [Google Scholar]
- Medin N. I., Osebold J. W., Zee Y. C. A procedure for pulmonary lavage in mice. Am J Vet Res. 1976 Feb;37(2):237–238. [PubMed] [Google Scholar]
- Parker J. C., Whiteman M. D., Richter C. B. Susceptibility of inbred and outbred mouse strains to Sendai virus and prevalence of infection in laboratory rodents. Infect Immun. 1978 Jan;19(1):123–130. doi: 10.1128/iai.19.1.123-130.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raut S., Hurd J., Blandford G., Heath R. B., Cureton R. J. The pathogenesis of infections of the mouse caused by virulent and avirulent variants of an influenza virus. J Med Microbiol. 1975 Feb;8(1):127–136. doi: 10.1099/00222615-8-1-127. [DOI] [PubMed] [Google Scholar]
- SCHEEL L. D., DOBROGORSKI O. J., MOUNTAIN J. T., SVIRBELY J. L., STOKINGER H. E. Physiologic, biochemical, immunologic and pathologic changes following ozone exposure. J Appl Physiol. 1959 Jan;14(1):67–80. doi: 10.1152/jappl.1959.14.1.67. [DOI] [PubMed] [Google Scholar]
- Smith H. Mechanisms of virus pathogenicity. Bacteriol Rev. 1972 Sep;36(3):291–310. doi: 10.1128/br.36.3.291-310.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D. J., Lebowitz M., Cassell E. J., Wolter D., McCarroll J. Health and the urban environment. 8. Air pollution, weather, and the common cold. Am J Public Health Nations Health. 1970 Apr;60(4):731–739. doi: 10.2105/ajph.60.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilma T., Zee Y. C., Osebold J. W. Immunofluorescence determination of the pathogenesis of infection with influenza virus in mice following exposure to aerosolized virus. J Infect Dis. 1979 Apr;139(4):458–464. doi: 10.1093/infdis/139.4.458. [DOI] [PubMed] [Google Scholar]
- Zee Y. C., Osebold J. W., Dotson W. M. Antibody responses and interferon titers in the respiratory tracts of mice after aerosolized exposure to influenza virus. Infect Immun. 1979 Jul;25(1):202–207. doi: 10.1128/iai.25.1.202-207.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]




