Abstract
The life-history strategies of organisms are sculpted over evolutionary time by the relative prospects of present and future reproductive success. As a consequence, animals of many species show flexible behavioral responses to environmental and social change. Here we show that disruption of the habitat of a colony of African cichlid fish, Haplochromis burtoni (Günther) caused males to switch social status more frequently than animals kept in a stable environment. H. burtoni males can be either reproductively active, guarding a territory, or reproductively inactive (nonterritorial). Although on average 25–50% of the males are territorial in both the stable and unstable environments, during the 20-week study, nearly two-thirds of the animals became territorial for at least 1 week. Moreover, many fish changed social status several times. Surprisingly, the induced changes in social status caused changes in somatic growth. Nonterritorial males and animals ascending in social rank showed an increased growth rate whereas territorial males and animals descending in social rank slowed their growth rate or even shrank. Similar behavioral and physiological changes are caused by social change in animals kept in stable environmental conditions, although at a lower rate. This suggests that differential growth, in interaction with environmental conditions, is a central mechanism underlying the changes in social status. Such reversible phenotypic plasticity in a crucial life-history trait may have evolved to enable animals to shift resources from reproduction to growth or vice versa, depending on present and future reproductive prospects.
Animals can use different behavioral strategies depending on immediate social and environmental conditions. Shifts in strategy are thought to reflect the likelihood of present and future reproductive success (1, 2). Because reproductive prospects depend on many factors, including changes in fecundity, longevity, and population structure, to change strategy, animals must be able to assess current reproductive opportunities and respond by changing phenotype. For example, a male may delay reproduction if he cannot compete with currently superior/dominant animals. Decisions about such life-history strategies may be made by changes in growth rate in some species (see, e.g., refs. 3–6). In these species, typically displaying indeterminate growth, the allocation of resources toward somatic growth and hence future reproductive success occurs only once in the life of an animal. However, in the African cichlid fish, Haplochromis burtoni (Günther), there is a socially regulated dynamic response to reproductive opportunity that depends ultimately on animal size.
H. burtoni is a nonseasonal breeder that lives in shallow temporary shorepools and estuaries of Lake Tanganyika in tropical East Africa (7, 8). In the field, 20–40% of the adult male population are territorial (T) at any time; they exhibit bright body coloration, maintain territories, and have mature testes. Only Ts are reproductively active, whereas the remaining nonterritorial males (NT) school with females, are cryptically colored, and are sexually regressed (7). These characteristics are seen also in laboratory populations (9). It has been demonstrated previously that a change is social status causes a change in the size of GnRH-containing neurons of the hypothalamus (10) and in the GnRH mRNA content of this brain region (S. A. White and R.D.F., unpublished work). However, these differences between T and NT males have been measured in stable environmental and social situations whereas in the natural habitat conditions fluctuate significantly (8). For example, strong daily winds deliver natural debris that interrupts the three-dimensional layout of the shallow habitats. Moreover, T males are much more likely to be targets of predation because of their conspicuously bright coloration (7), and hippopotami (Hippopotamus amphibius) traversing colonies disrupt territorial structures (8).
To understand whether and how environmental instability influences the social system, we subjected colonies of H. burtoni to systematic alterations. Animals in a fluctuating habitat undergo more frequent changes in social status and, importantly, these status changes regulate somatic growth rate. The regulated growth of individuals unexpectedly acts to stabilize the social scene in the short term while facilitating social upheaval in the long term.
Materials and Methods
To discover the effects of environmental change on the social status of individuals, we subjected colonies of animals to one of two conditions: fluctuating conditions, which simulate alterations in the natural environment, and stable conditions. In both conditions, the behavior of the animals was observed and their growth rate measured.
Animals.
Fish derived from a wild-caught stock population were kept in aquaria under conditions similar to those of their natural environment (8): pH 8; 28°C water temperature; 12/12-hr light/dark cycle with full-spectrum illumination. Gravel covered the floors of the aquaria, and terracotta pots on the substrate facilitated the establishment and maintenance of territories necessary for successful reproduction (7). Fish were fed every morning ad libitum with cichlid pellets and flakes (Aquadine, Healdsburg, CA). All work was in compliance with the Animal Care and Use guidelines at Stanford University.
Experimental Design.
Individually tagged males (10–14 fish per tank) from different age cohorts (average lengths per tank ranged from 4.9 to 9.2 cm; standard deviations ranged from 0.1 cm to 0.9 cm) were placed in 100-liter aquaria [91 × 45 × 25 cm] with approximately the same numbers of females. The standard lengths of the fish were measured weekly or biweekly. This interval between handling was considered sufficient for recovery from the associated stress because fish displayed their normal behaviors less than 1 hr after the measurements. To allow for statistical analysis, growth rates were calculated as the relative change in standard length over a 7-day period.
Fluctuating Condition.
Changes in the local environment, analogous to those observed in the field after disruption of the habitat by hippopotami, winds, and predation (7), were simulated by systematically changing the arrangement of bottom cover in four aquaria over a period of 18–22 weeks. Terracotta pots, defended by territorial males, were arranged in four distinct patterns in each of four different tanks (see Fig. 1). Nothing else (e.g., amount and distribution of gravel, filters, etc.) was changed. Standard lengths of all males were measured each time the pot layout was changed.
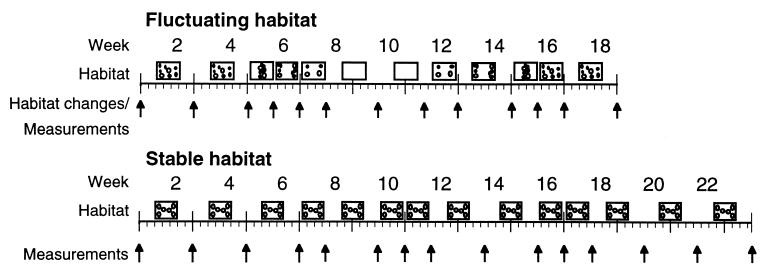
Figure 1.
Schematic representation of the time sequence of the experiment showing the two conditions that differ in the arrangement of the substrate: fluctuating or stable. Rectangles indicate the aquarium floor with small circles representing terracotta pots used to provide cover for animals. In the fluctuating condition, pots were rearranged in either a random fashion or concentrated in the center, edges, or corners of the tank, or there were no pots at all, as shown schematically. In the stable condition, pot arrangement was kept constant. Arrows indicate when the substrate was altered and fish were measured.
Stable Condition.
As a control condition, two aquaria were maintained unchanged for the entire experimental period (20–23 weeks). The flower pots were arranged with one pot in each corner and two half pots in the center facing toward the left and right edge, respectively (see Fig. 1).
Behavioral Observations.
All animals were observed at least three times per week for 20 min between 1000 and 1300 hours. Ts and NTs were categorized based on their characteristic coloration and behavioral patterns (9): Brightly colored T males display a black lachrymal stripe across the eyes, are more aggressive (chasing or biting; threat displays; border conflicts and fights with other Ts) and are sexually active (digging of gravel, courting, spawning). In contrast, the sandy gray-colored NTs tend to form schools and flee from chasing Ts. Animals in transition between those two states alternated coloration patterns and behaviors typical of both categories of males. The location of the territories within each tank was also recorded.
Statistics.
Values presented are means ± standard error, unless stated otherwise. Parametric or nonparametric tests for statistical significance were performed depending on the fulfillment of the normality condition by using gbstat software (Dynamic Microsystems, Silver Spring, MD). Sets of frequencies were compared with Cochran’s Q test.
Results
Social Stability.
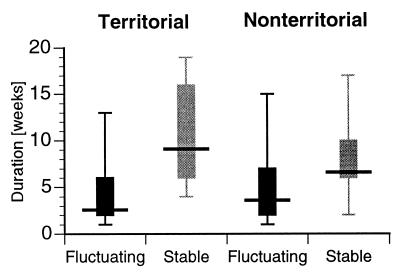
Fluctuations in the habitat induced changes in male social status (Fig. 2A). In striking contrast, the frequency of social change was substantially lower in a stable habitat (Fig. 2B). However, even in the stable habitat, a number of fish changed status during the course of the experiment. Correspondingly, territorial tenure duration was decisively shorter in all four tanks with fluctuating habitat (median: 3 weeks; interquartile range: 2–6 weeks; n = 52; Fig. 3) and in this condition, no individual maintained T status throughout the experiment. Correspondingly, time spent as NT averaged only 4 weeks in the fluctuating condition (interquartile range: 2 to 7 weeks; n = 55). As expected, T males in the two stable habitat tanks had significantly longer tenure (median: 9.5 weeks; interquartile range: 6–16 weeks; n = 14), including some males that remained T for the whole experiment. Time as NT was 7 weeks on average (interquartile range: 6–10 weeks; n = 15). These differences in the duration of territoriality (two-group comparison after Kolmogorov-Smirnov: P < 0.001) and nonterritoriality (P < 0.05) are significant between the two conditions.
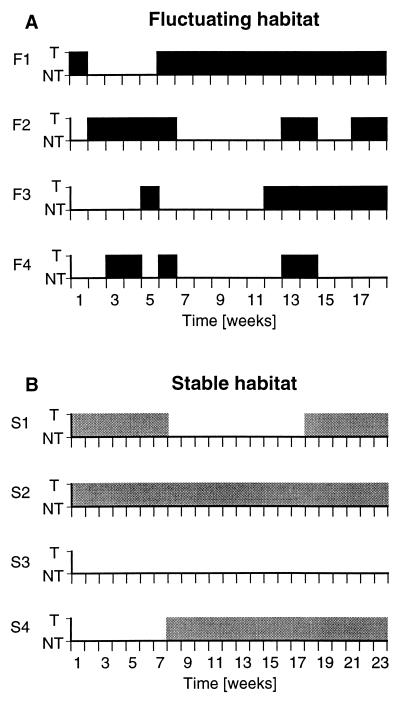
Figure 2.
Social status as a function of time for representative animals from the two experimental conditions (T, territorial; NT, nonterritorial). (A) As is evident, the fluctuating environmental conditions resulted in frequent changes of social status in most animals. Note that the animal in the top graph (F1) remained territorial for a long period of time despite environmental changes. (B) Stable environmental conditions resulted in relatively longer periods of territorial tenure, although changes still occurred.
Figure 3.
Representation of time spent as either territorial or nonterritorial under fluctuating and stable conditions, respectively. Shown are the median (dark horizontal bar) and interquartile ranges (thick vertical bars), as well as minimum and maximum. The median duration of territoriality is 3 weeks in the fluctuating condition (n = 52) and 9.5 weeks in the stable condition (n = 14). In contrast, the median time spent as nonterritorial is only 4 weeks in the fluctuating condition (n = 55), compared with 7 weeks under stable conditions (n = 15).
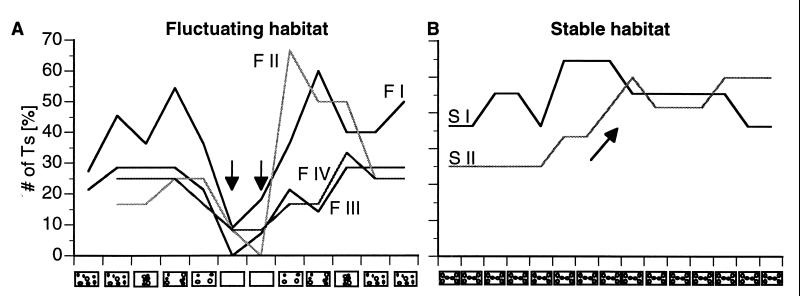
For a few days after a change in the habitat, Ts as well as many NTs displayed more aggressive interactions until fish had settled again into their new social roles. In the fluctuating condition, the proportion of males that established or maintained a territory after change varied widely in some tanks (Fig. 4A). However, this variability was statistically significant in only one of the four tanks (Cochran’s Q test: Tank F I: P = 0.1664; Tank F II: P < 0.005; Tank F III: P = 0.6887; Tank F IV: P = 0.6445). Interestingly, while two tanks showed a surge of territorial activity after the reintroduction of shelters, the other two tanks took longer to recover. Consistently, an environment devoid of cover sustained only a few territories (Fig. 4A, arrows), presumably because of the lack of adequate shelters. Under stable habitat conditions, the proportion of Ts was less variable (Fig. 4B). Although one tank showed a sustained increase in the proportion of Ts halfway through the experiment (Fig. 4B, arrow) when a number of NTs were able to establish territories for the first time, this change was not significant (Cochran’s Q test: Tank S I: P = 0.9246; Tank S II: P = 0.4625).
Figure 4.
Percentage of Ts in relation to the structure of the habitat. (A) In the fluctuating habitat (tanks F I to F IV), the lack of shelters suitable for territories dramatically reduces the number of Ts (arrows). Highly structured habitats, however, can sustain significantly more Ts. (B) Stable habitat conditions (tanks S I to S II) show less variation in the proportion of Ts, although it appears to increase over time when more and more NTs turn T with only a few Ts losing their territories (arrow).
Interestingly, the proportion of males that became T at least once in the course of an experiment was not different between the fluctuating (59.2%, n = 73) and stable conditions (65.2%, n = 25; P > 0.05, χ2 test; Fig. 5). This means that the proportion of males that became territorial for at least one observation interval increased continuously over the experimental period. These data predict that every male will have been reproductively active (T) at least once within a year.
Figure 5.
Cumulative frequency of males that were territorial for at least 1 week over the course of the experiments. In both conditions, almost two-thirds of the male population became territorial for at least 1 week and thus had a chance to reproduce (fluctuating habitat, 59.2%, n = 49; stable habitat, 65.2%, n = 23; not significant with χ2 test). Linear regression analysis yields y = 2.10x + 23.43 (r2 = 0.91; P < 0.0001) for the fluctuating condition, and y = 1.89x + 28.68 (r2 = 0.85; P < 0.0001) for the stable condition.
Growth Rates.
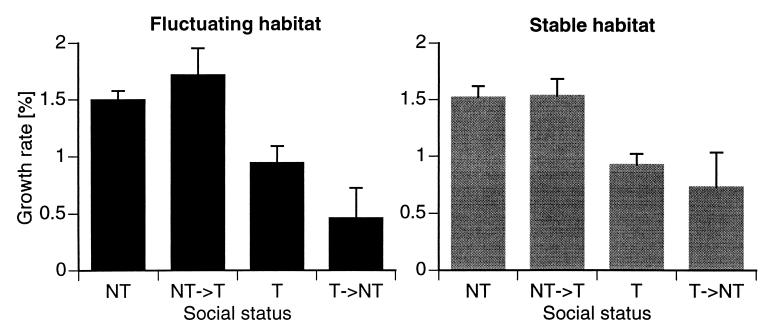
To our great surprise, the growth rates of males in the four social categories (Ts, NTs, descending males: T→NTs; ascending males: NT→Ts) were distinctly different (ANOVA: fluctuating, P < 0.0001; stable, P < 0.0002). In all tanks, in both stable and fluctuating habitats, NT→Ts (i.e., males that had become T ≈1 week earlier) grew more than average whereas T→NTs (who had lost territoriality 1 week earlier) grew less than average or even shrank. In two representative examples for each condition, the dependence of growth on social status is evident (Fig. 6). Growth rates were significantly different within each experimental condition (Fig. 7). NTs grew faster than Ts (group comparisons after Duncan: P < 0.01 in both conditions) and descending (T→NT) males (fluctuating, P < 0.01; stable, P < 0.05). Ascending animals also grew faster than Ts (P < 0.05 in both conditions) and descending (T→NT) males (fluctuating, P < 0.01; stable, P < 0.05). Differences between NTs and ascending fish were not significant, and neither were differences between Ts and descending males.
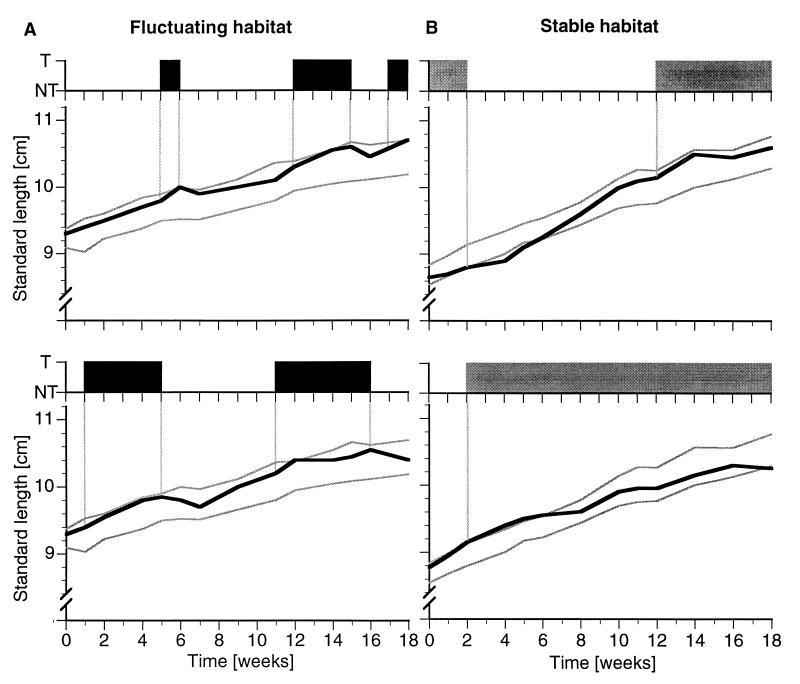
Figure 6.
Standard lengths and social histories of representative individuals from fluctuating (A) and stable (B) environments. The top of each panel shows the social status of the respective animals over the course of the experiment (T, territorial; NT, nonterritorial). At the bottom of each panel, the standard lengths (black line) of this individual and the standard deviations (gray lines) of the size distributions of the respective male population are shown. In both conditions, animals grow faster while they are NT and often slow down while they are T, as can be seen by the different slopes. Standard length typically increases when an animal becomes territorial (NT→T) and often decreases when it becomes nonterritorial (T→NT).
Figure 7.
Statistical summaries of average growth rates (± standard error) for four distinct social classes of animals under fluctuating (A) and stable (B) conditions. Growth rates are statistically significantly different within each experimental condition (one-way ANOVA; fluctuating, P < 0.0001; stable, P < 0.0002). NTs grow faster than Ts and descending (T→NT) males. Ascending (NT→T) animals also grow faster than Ts and descending males.
Discussion
Clearly, environmental change facilitates changes in social status, and social status regulates growth in H. burtoni. Such phenotypic plasticity allows, in principle, every male fish to become territorial and, hence, to reproduce. Differential growth rates enable individuals to allocate resources appropriately for current status but also to be prepared for reproductive opportunities.
It is remarkable that habitat complexity or, more exactly, shelter availability has such a pronounced impact on the territorial density and, hence, on male reproductive opportunity. Complex habitats evidently offer more possibilities for males to establish territories, probably because of greater visual isolation among the Ts. In the natural environment, T males prefer highly structured habitats and maintain adjacent territories when these are visually isolated (7). However, if the isolation changes (e.g., a banana leaf moves), a vicious fight might follow that usually results in one animal withdrawing (7). Field data also show that predation by birds is an important factor inducing social change in H. burtoni, mostly affecting territorial fish, probably because of their conspicuous coloration (8, 11). After a T male has been removed, vigorous fighting over the vacant territory ensues (7). In addition, it has been observed in the field that strong daily winds can lead to considerable change of the three-dimensional layout of the habitat, which often results in Ts shifting their territories and thus opening vacant space for NTs to become T (7).
Male H. burtoni show striking differences in their reproductive physiology causally dependent on social status. It has been shown (T. Nguyen, S. A. White, and R.D.F., unpublished work) that H. burtoni males respond to experimentally induced changes in their social status asymmetrically: Fish ascending in social status gain reproductive function (i.e., the hypothalamic-pituitary-gonadal axis becomes activated) in ≈1 week whereas descending males maintain functional reproductive organs for up to 3 weeks after defeat. Because the median duration of territoriality under fluctuating conditions is 3 weeks, a quick up-regulation of the reproductive mechanisms after gaining T status is clearly desirable. Conversely, new opportunities for establishing territoriality occur at an average of 4 weeks; hence, descending males may benefit by maintaining their reproductive functions as long as possible. This reasoning is supported by the available field data (7). Although the number of Ts remains relatively stable over time, in a typical shorepool an average of two territories become vacant every day, partly because of predation (7). From that we estimate the duration of territorial tenure at ≈4 weeks on average whereas the mean duration of NT status is ≈7 weeks.
We conclude that there may be a trade-off between maintaining a reproductive state on the one hand and reallocating resources toward growth that increase the chances for a comeback on the other hand. In the limiting case, a highly unstable habitat, fish might never completely attain NT status and the corresponding physiology. In contrast, during more stable conditions, animals may remain NT while investing their energy into somatic growth, thus becoming ready for reproductive opportunities.
The lifespan of male H. burtoni under natural conditions is ≈18 months (7). Although H. burtoni males can establish territories and, hence, be sexually mature at an age of 3 months, maturation in juveniles is suppressed by the presence of larger adults (12, 13). Of ≈1,200 individuals found in one shore pool, ≈600 were males, but only ≈150 of them were large enough to hold a territory or to potentially switch from NT to T (R.D.F., unpublished data). The other 450 fish were juveniles of different size and age that were heavily predated on by water snakes (8). In addition, after the release from the mother’s mouth, young fry are often attacked and eaten by older conspecifics (R.D.F., unpublished observations). The fish surviving these assaults can be expected to join the potentially reproductive fraction of the population as NTs and may be aided in this pursuit by relatively high growth rates. Thus, there seems to be a continuous stream of fish developing from juveniles to adult NTs and ultimately maturing to reproducing Ts.
It is interesting that social status can have such an impact on the somatic growth of an animal. In both laboratory and field, the number and frequency of behaviors performed by Ts is significantly larger than in NTs (7, 9). In general, dominant animals are more active and, as a consequence, body reserves may get depleted over time (14). It is not clear whether H. burtoni territories are more than sites of mate attraction, or also serve as food resources (8). Thus, in the field, T males may be able to compensate for their increased energy expenditure by simply eating more. However, the structure of the natural H. burtoni habitat in shallow shorepools and estuaries of tropical Lake Tanganyika indicates that nutrients may not be limited, either in space or time (15). Field observations showed that NTs spend much more time feeding than Ts (7). Similarly, in two Mbuna cichlid species in Lake Malawi with generalist feeding habits and a social structure comparable to H. burtoni, NTs are in better physical condition than Ts (16). This evidence clearly reveals the possibility that NTs enhance their chance of becoming T by investing more in growth while waiting for disruptions in the habitat or predation on Ts.
From an evolutionary perspective, it is important to note that almost two-thirds of the males in our study gained territoriality at least once. This may explain why sneaking behavior, i.e., nonterritorial males trying to fertilize eggs in a dominant fish’s territory (17), has not been found in this species (7). If reproductive opportunities arise frequently, devoting energy to growth while NT may be a more effective strategy than sneaking, which carries a metabolic cost and the risk of being discovered by the residing male.
One of the foundations of life-history theory is the notion that subordinate animals can trade the benefit of immediate for future reproduction (1). As a consequence, age and size at maturity are often seen as phenotypically plastic (18). For example, in the platyfish, Xiphophorus variatus, adult size is under social control (3). In the pipefish Syngnathus typhle—in which the sex roles are reversed and females compete for males—the presence of a larger female reduces ovarian productivity and increases body growth (5). Similarly, small females of the dwarf surfperch Micrometus minimus delay reproductive activity in what can at least partly be seen as a tactical adaptation in the presence of larger females (6). Wickings and Dixson (19) showed that subordinate male mandrills, Mandrillus sphinx, can delay their sexual development during puberty until they find more favorable conditions. In these examples, however, animals usually change their behavioral strategy only once during their lifetime. In contrast, H. burtoni can change behavior as well as resource allocation frequently throughout life depending on the environmental and social situation. This reversible phenotypic plasticity seems highly adaptive because environmental and social changes occur at an interval shorter than generation time (20).
Although the reproductive benefits of territoriality and dominance are obvious, their costs are much more difficult to measure. Our work suggests that the allocation of energy away from growth is such a cost in H. burtoni. In addition, environmental instability imposes pressures onto the fish requiring quick behavioral as well as physiological responses when social opportunities arise or vanish. One such response is an increased level of circulating glucocorticoids (e.g., cortisol). In primates (21, 22) and birds (23), social instability as well as unpredictable environmental conditions can lead to increased levels of cortisol. As has been shown previously in a laboratory population of H. burtoni (24), cortisol levels are similar in Ts and NTs as long as the social scene is unstable. Moreover, animals losing territories often have extremely high levels of this hormone, indicating that defeat results in a significant endocrine response. Conversely, ascending fish often exhibit lower cortisol levels unless they are unable to sustain the newly acquired territory (24). Interestingly, under conditions in which virtually no social change occurs, NTs have elevated cortisol levels when compared with Ts (24). In light of our present data, we interpret this result to mean that NTs that are ready to become T but lack the opportunity exhibit a physiological stress response to this social situation. However, because even in a stable condition social change occurred, the elevated cortisol levels may not be sufficient to suppress growth. To test these hypotheses, we are currently examining the stress response as well as social behavior and growth rates under both fluctuating and stable conditions. Although defeated animals often slow their growth, possibly via the action of cortisol (25), they exhibit an environmental optimism by maintaining their reproductive capabilities as long as possible (T. Nguyen, S. A. White, and R.D.F., unpublished work). Later, as established NTs, they invest in future reproduction by allocating resources again toward growth. The study of this phenotypic plasticity in life-history traits opens up exciting new ways of investigating both the ultimate and proximate effects of social behavior.
Acknowledgments
We thank A. Ettinger, K. Hoke, W. Loher, R. Robison, and R. White for comments on earlier versions of the manuscript. This work was supported by the Deutsche Forschungsgemeinschaft (H.A.H.) and National Institutes of Health Grant NS-34950 (R.D.F.).
Abbreviations
- T
territorial males
- NT
nonterritorial males
References
- 1.Williams G C. Adaptation and Natural Selection. Princeton, NJ: Princeton Univ. Press; 1966. [Google Scholar]
- 2.Stearns S C. In: Dahlem Konferenzen: Evolution and Development. Bonner J T, editor. Berlin: Springer; 1982. pp. 237–258. [Google Scholar]
- 3.Borowsky R. Nature (London) 1973;245:332–335. [Google Scholar]
- 4.Metcalfe N B, Huntingford F A, Graham W D, Thorpe J E. Proc R Soc London Ser B. 1989;236:7–19. doi: 10.1098/rspb.1989.0009. [DOI] [PubMed] [Google Scholar]
- 5.Berglund A. Evolution (Lawrence, Kans) 1991;45:770–774. doi: 10.1111/j.1558-5646.1991.tb04346.x. [DOI] [PubMed] [Google Scholar]
- 6.Schultz E T, Clifton L M, Warner R R. Am Nat. 1991;138:1408–1430. [Google Scholar]
- 7.Fernald R D, Hirata N R. Anim Behav. 1977;25:964–975. [Google Scholar]
- 8.Fernald R D, Hirata N R. Env Biol Fish. 1977;2:299–308. [Google Scholar]
- 9.Fernald R D. Anim Behav. 1977;25:643–653. [Google Scholar]
- 10.Francis R C, Soma K, Fernald R D. Proc Natl Acad Sci USA. 1993;90:7794–7798. doi: 10.1073/pnas.90.16.7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haas R. Evolution (Lawrence, Kans) 1976;30:614–622. doi: 10.1111/j.1558-5646.1976.tb00938.x. [DOI] [PubMed] [Google Scholar]
- 12.Fraley N B, Fernald R D. Z Tierpsychol. 1982;60:66–82. [Google Scholar]
- 13.Davis M R, Fernald R D. J Neurobiol. 1990;21:1180–1188. doi: 10.1002/neu.480210804. [DOI] [PubMed] [Google Scholar]
- 14.Bowen S H. J Fish Biol. 1984;24:115–121. [Google Scholar]
- 15.Coulter G W. Lake Tanganyika and Its Life. Oxford: Oxford Univ. Press; 1991. [Google Scholar]
- 16.Munthali S M. J Appl Ichthyol. 1996;12:131–134. [Google Scholar]
- 17.Sinervo B, Lively C M. Nature (London) 1996;380:240–243. [Google Scholar]
- 18.Stearns S C, Koella J C. Evolution (Lawrence, Kans) 1986;40:893–913. doi: 10.1111/j.1558-5646.1986.tb00560.x. [DOI] [PubMed] [Google Scholar]
- 19.Wickings E J, Dixson A F. Physiol Behav. 1992;52:909–916. doi: 10.1016/0031-9384(92)90370-h. [DOI] [PubMed] [Google Scholar]
- 20.Bradshaw A D. Adv Genet. 1965;13:115–155. [Google Scholar]
- 21.Sapolsky R M. Am J Primatol. 1983;11:217–226. doi: 10.1002/ajp.1350110303. [DOI] [PubMed] [Google Scholar]
- 22.Alberts S C, Sapolsky R M, Altmann J. Horm Behav. 1992;26:167–178. doi: 10.1016/0018-506x(92)90040-3. [DOI] [PubMed] [Google Scholar]
- 23.Wingfield J C, Ramenofsky M. Ardea. 1997;85:155–166. [Google Scholar]
- 24.Fox H E, White S A, Kao M H F, Fernald R D. J Neurosci. 1997;17:6463–6469. doi: 10.1523/JNEUROSCI.17-16-06463.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pickering A D. In: Progress in Clinical and Biological Research, Vol. 342: Progress in Comparative Endocrinology. Epple A, Scanes C G, Stetson M H, editors. New York: Wiley–Liss; 1990. pp. 473–479. [Google Scholar]