Abstract
Due to its unique physicochemical and optical properties, C60 has raised interest in commercialization for a variety of products. While several reports have determined this nanomaterial to act as a powerful antioxidant, many other studies have demonstrated a strong oxidative potential through photoactivation. To directly address the oxidative potential of C60, the effects of light and chemical supplementation and depletion of glutathione (GSH) on C60-induced toxicity were evaluated. Embryonic zebrafish were used as a model organism to examine the potential of C60 to elicit oxidative stress responses. Reduced light during C60 exposure significantly decreased mortality and the incidence of fin malformations and pericardial edema at 200 and 300 ppb C60. Embryos co-exposed to the glutathione precursor, N-acetylcysteine (NAC), also showed reduced mortality and pericardial edema; however, fin malformations were not reduced. Conversely, co-exposure to the GSH synthesis inhibitors, butathionine sulfoximine (BSO) and diethyl maleate (DEM), increased the sensitivity of zebrafish to C60 exposure. Co-exposure of C60 or its hydroxylated derivative, C60(OH)24, with H2O2 resulted in increased mortality along the concentration gradient of H2O2 for both materials. Microarrays were used to examine the effects of C60 on the global gene expression at two time points, 36 and 48 hours post fertilization (hpf). At both life stages there were alterations in the expression of several key stress response genes including glutathione-S-transferase, glutamate cysteine ligase, ferritin, α-tocopherol transport protein and heat shock protein 70. These results support the hypothesis that C60 induces oxidative stress in this model system.
Keywords: fullerene, photoactivation, gene expression
Introduction
Carbon fullerenes, otherwise known as Buckminster fullerenes, or “bucky balls”, have unique physicochemical properties that may be exploited for use in consumer products such as cosmetics, lubricants, food supplements, building materials, clothing treatment, electronics and fuel cells (Loutfy et al., 2002). While fullerenes are currently produced by the ton every year, actual commercialization is still mostly under development (Robichaud et al., 2005). Due to the potential for widespread applications and consequently, widespread exposures, evaluation of the biological effects of these important nanomaterials is warranted. C60 has been shown to induce toxicity in numerous cell culture and whole animal systems (Oberdorster, 2004; Sayes et al., 2005; Isakovic et al., 2006; Usenko et al., 2007). However, contradictory reports in the literature make it difficult to interpret the mechanism by which C60 toxicity is induced.
Fullerene C60 has been described as a free radical scavenger by some (Dugan et al., 1996; Wang et al., 1999; Mori et al., 2006) yet others have reported it to generate oxygen free radicals within and outside of biological systems (Yamakoshi et al., 2003; Isakovic et al., 2006). With few exceptions, pristine C60 and suspensions of C60 clusters (nC60) are reportedly more toxic than their derivatives (Isakovic et al., 2006). In cell culture, nC60 synergized the effects of oxidative stress-inducing agents and elicited cytotoxic action through cell membrane lipid peroxidation (Sayes et al., 2005; Isakovic et al., 2006). In vivo, nC60 induced oxidative stress and lipid peroxidation in the brain of juvenile largemouth bass (Oberdorster, 2004). However, others have reported C60 to act as a powerful antioxidant in vivo in rats with no acute or subacute toxicities (Gharbi et al., 2005). Certain surface modifications (e.g. hydroxylation) have been shown to impart cytoprotective activity by eliminating reactive oxygen species (ROS) and antagonizing the effects of the oxidative stress-dependent cytotoxicity (Dugan et al., 2001; Bogdanovic et al., 2004; Isakovic et al., 2006). In fact, carboxylated-C60 has been patented as a method to increase a metazoan’s lifespan presumably through these same mechanisms (Dugan et al., 2003). Despite disagreement amongst researchers as to the oxidative potential of C60, oxidative stress is currently the leading proposed mechanism through which fullerenes may induce toxicity.
Oxidative stress is a common pathway of toxicity and disease. An organism can undergo oxidative stress through several different mechanisms. First, it may be directly induced by an oxidizing agent, such as H2O2. Second, it may be produced through the induction of cytochrome P450, as is the case with many polycyclic aromatic hydrocarbons (PAHs) (Wassenberg and Di Giulio, 2004). Third, a xenobiotic may inhibit the production of antioxidant molecules, such as glutathione (GSH), that function to maintain oxidative balance (Anderson, 1998). GSH is an endogenous tripeptide enzyme and known free radical scavenger, thus, it is important for detoxification of metabolites and ROS associated with chemical exposure and disease (Asikainen et al., 2002; Franklin et al., 2002; Suh et al., 2004). It has yet to be determined through which, if any, of these pathways fullerenes may act.
The physicochemical properties of C60 support the hypothesis that this nanomaterial may induce oxidative stress following photoactivation. Due to its unique spherical structure, C60 has the ability to accept up to 6 electrons (Jensen et al., 1996). These electrons essentially race around the structure through dipole moments. When C60 is acted upon by light, it is raised to a higher energy level producing singlet C60, which reacts with O2 to form singlet oxygen (1O2) (Hirsch and Brettreich, 2005). The amount of UV absorption necessary to raise the molecule to the triplet state varies with surface functionalization (Prat et al., 1999). In general, more functional groups added to the fullerene requires more energy to move to the excited state, resulting in lower triplet and singlet oxygen quantum yields (Prat et al., 1999). In the presence of C60, both visible and ultraviolet light can generate ROS, particularly as singlet oxygen and superoxide (Pickering and Wiesner, 2005). These byproducts can induce oxidative stress leading to a variety of detrimental downstream effects such as lipid peroxidation, DNA and protein adduction and cellular death (Kamat et al., 2000; Dugan et al., 2001; Dhawan et al., 2006).
In these studies, we exploit the numerous advantages of the embryonic zebrafish model to elucidate the mechanism by which C60 induces its toxic actions. This model has been used extensively for drug discovery and chemical or nanomaterial screening to rapidly evaluate integrated system effects and subsequently identify mechanisms of toxic action (Usenko et al., 2007). Here, we tested the effect of light activation and antioxidant environment on C60 toxic potential, and used zebrafish microarrays to evaluate global gene expression following C60 exposure. Further, we evaluated both C60 and hydroxylated C60 for antioxidative protection from H2O2 exposure. The results presented herein strongly indicate oxidative stress as a pathway of C60-induced toxicity in this experimental model.
Methods
Solution Preparation
C60 was obtained from Sigma Aldrich, WI (99+%) and dissolved in 100% dimethyl sulfoxide (DMSO). The stock solution of 50 ppm C60 was sonicated for 1.5 hours to ensure a uniform dispersion and size distribution. C60(OH)24 (MER Corp, Arizona) was dissolved in DMSO and sonicated for 5 minutes to ensure uniform distribution. The size range of C60 and C60(OH)24 agglomerates following sonication were measured using photon correlation spectroscopy and previously reported in Usenko et al., 2007. H2O2 was purchased from VWR International (Brisbane, CA) and was diluted to make exposure solutions of 0.5 mM and 1.0 mM. Buthionine sulfoximide (BSO), diethyl maleate (DEM), N-acetylcysteine (NAC), and L-glutathione (GSH) were purchased from Sigma Aldrich (St. Louis, MO).
General Exposure Protocol
Embryonic zebrafish were obtained from the AB strain of zebrafish (Danio rerio) reared in the Sinnhuber Aquatic Research Laboratory (SARL) at Oregon State University. Adults were kept at standard laboratory conditions of 28 °C on a 14 h light/10 h dark photoperiod with a conductivity of 500 Siemens per meter. Water consisted of reverse osmosis (RO) water supplemented with a commercially available salt solution (0.6% Instant Ocean®). Zebrafish were spawned and embryos were collected and staged (Reimers et al., 2006). The chorion, an acellular envelope surrounding the embryo, was removed via enzymatic reaction with pronase at 24 hours post fertilization (hpf) as described previously (Usenko et al., 2007). At 24 hpf, embryos were placed individually into wells of a 96-well plate, so that there was one embryo per well, and exposed to 100 μl of nanomaterial solution.. A 1% DMSO in water solution was used as a control as this concentration was previously found to have no effect on the embryos. Embryos were evaluated every 24 hours for 5 days for morphological malformations, mortality and behavioral abnormalities.
Dark Exposure
To determine if light influenced the toxic potential of C60, the stock solution was prepared and kept in the dark and the 96-well plates were protected from the light after exposure. Embryos were dechorionated at 24 hpf and placed individually in wells and exposed to 1% DMSO controls, 100, 200, 300, or 500 ppb C60 (equivalent to 0, 0.05, 0.1, 0.15, 0.25 ng/mg as reported in Isaacson et al., 2007). Embryos were analyzed daily until 120 hpf for physiological malformations and mortality. The cumulative results were reported for effects. Sublethal effects were scored against number of surviving animals at the initial assessment (24 hours of exposure).
Co-incubation with GSH level-modifying Chemicals
The concentration-response of each of the test chemicals was conducted to determine the levels at which there was an adverse effect. Embryos were dechorionated at 24 hpf and were scored daily until 120 hpf. Embryos were co-incubated with either 100 μM GSH, 50 μM NAC, 250 μM ascorbic acid, 5 μM BSO, or 50 nM DEM. Previous reports found the maximum tolerable concentration (MTC) ascorbic acid to be 250 μM and GSH to be 100 μM (Hirsch and Brettreich, 2005). A concentration gradient was used for other co-incubations in this study. NAC is the rate-limiting substrate in GSH synthesis, and thus was used to potentially increase GSH production. BSO and DEM were used to inhibit GSH synthesis, thus making the embryos more sensitive to oxidative stressors. All chemicals were co-incubated with 50–300 ppb C60 or 1% DMSO and evaluated as described above. The pH of exposure solutions was monitored and buffered appropriately. H2O2 is a known oxidant and was co-incubated with 10 ppb C60 or 100 ppb C60(OH)24 to determine if either fullerene shifted the concentration-response curve to the right or left. H2O2 was co-incubated at 0.5 mM and 1.0 mM based on initial concentration-response of H2O2. At 1.0 mM, H2O2 induced 100% mortality.
Cellular Death Assays
Cellular death was evaluated in embryos co-exposed to C60 and NAC, DEM, BSO or ascorbic acid. This endpoint serves as an early indicator of perturbation based on previous evaluations that showed significant cell death could be detected after only 12 hours of exposure to C60 (Reimers et al., 2006). Since cellular death was evaluated in whole embryos, potential targets of toxi city could be identified. Embryos were exposed to 100 ppb C60 or 1% DMSO and were co-exposed to 50 μM NAC, 50 nM DEM, 5 μM BSO, or 250μM ascorbic acid at 24 hpf. At 36 hpf, embryos were rinsed with water and then incubated in 100 μl of 5 μg/ml acridine orange for one hour in the dark at 28 °C. Embryos were rinsed again, mounted in low melt agarose (1% w/v, Promega, Madison, WI) and imaged using an Axiovert 200M Zeiss microscope (Carl Zeiss International, Germany) with a 546 nm filter. Fluorescence in the head region was measured and quantified using ImagePro Plus software (Media Cybernetics, Inc., Silver Spring, MD).
Global Gene Expression Analysis
Custom Array Chip
Oligonucleotides (50 mer) were purchased from MWG (High Point, NC). Zebrafish custom arrays of 14,000 genes were spotted by Eric Johnson at University of Oregon. Epoxy-coated slides were cross-linked and were kept at room temperature in a dessicator until use.
Isolation of RNA
Embryos were exposed to 200 ppb C60 or 1% DMSO at 24 hpf until 36 or 48 hpf and then pooled into three groups (biological replicates) of 40 embryos. Embryos were euthanized with tricaine methanesulfonate and rinsed thoroughly. Excess water was removed from the samples and 100 μl of TRIzol Reagent was added to extract RNA. Embryos were homogenized using a pestle on ice and stored at −80 °C until processing. Once all samples were collected, homogenates were thawed and 900 μl of TriReagent was added and each sample was vortexed. Next, the homogenates were centrifuged at 12,000 g at 4 °C, and then 200 μl chloroform was added. The samples were centrifuged again and the supernatants aliquoted into new vials. 500 μl of isopropanol was added to each sample and the sample was centrifuged. All liquid was removed and the pellet was washed several times with 70% ethanol: H2O. The pellets were air dried and then resuspended in 30 μl diethylpyrocarbonate-water. The extractions were flash frozen using liquid nitrogen and stored at −80 °C. RNA was quantified using NanoDrop sensor and the quality verified using Agilent’s BioAnalyzer 2100 (Palo Alto, CA) at the Center for Genome Research and Biocomputing (CGRB) at OSU.
Processing
Labeling, hybridization and scanning were performed at the CGRB. A Genisphere 950 labeling kit (Hatfield, PA) was the hybridization label for the samples. A dye swap for each sample was done to eliminate variability due to dye affinities. One microgram of RNA of each sample was reverse-transcribed with Superscript II (Invitrogen) using the Genisphere olido d(T) primer containing a capture sequence for the Cy3 or Cy5 labeling reagents. Slides were scanned using a GenePix Scanner at 543 nm for Cy3 and 633 for Cy5 at 80% power. Data was compiled using Gene Pix Software.
Data Analysis
Values from corresponding dye swaps were averaged between individual samples to obtain a single value for each sample. Treatments were conducted in triplicate for significance validation. Image files were quantified using QuantArray (PerkinElmer) and the raw data was imported into BASE software. Raw mean backgrounds were subtracted using LOWESS in order to eliminate dye-related artifacts. Genes induced or repressed by greater than 2-fold in all three replicates were selected and placed into categories based on function.
qPCR
Oxidative stress response genes were validated using quantitative real time polymerase chain reaction (qRT-PCR). cDNA was made from the RNA isolated for the microarrays. Briefly, cDNA was synthesized from 1 μg of total RNA per group using Superscript II (Life Technologies, Gaithersburg, MD) and oligo(dT) primers in a 20 ml volume. Primers were designed and ordered from MWG (High Point, NC) for several targeted genes (sequences given in table 1). Quantitative PCR was conducted using gene specific primers with the Opticon-2 real-time PCR detection system (MJ Research, Waltham, MA). For each PCR reaction, 1 μl of cDNA was used in the presence of SYBR Green, using DyNAmo SYBR green qPCR kit according to the manufacturer’s instructions (Finnzymes, Espoo, Finland). A temperature gradient (54 °C–58 °C) was used to determine the appropriate annealing temperature for each primer set. All primers had an annealing temperature of 58 °C and ran for 35 cycles. The PCR product was separated using gel electrophoresis to ensure only one product was made. The PCR product was mixed with 6x SDS dye and loaded onto a 1.5% agarose gel with 0.05% ethidium bromide. The gel electrophoresis was run at 110 V and 80 mA for 1 hour. The gel was viewed on an Ultraviolet Transilluminator and imaged with a Polaroid photo documentation camera. PCR data was analyzed against an adult cDNA calibrator using the GAPDH primer set by the Opticon Monitor 3.1 software (MJ Research, Waltham, MA). Each sample PCR product was analyzed against a glyceraldehydes-3-phosphate dehydrogenase (GAPDH) control for that sample.
Table 1.
Primer sequences used in quantitative PCR. All products are between 180 and 300 mer.
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Ferritin heavy chain | 5′-AGACACACTACTTGGACGAG | 5′-AACAAGCTAGGAGGTTCTGC |
| HSP70 | 5′-GACCAAAGACAACAACCTGC | 5′-ATGTTGAAGGCGTAAGACTCC |
| GCLc | 5′-CTATCTGGAGAACATGGAGG | 5′-CATTTTCCTCTGTTGACCGG |
| GAPDH | 5′-GAATTCTGGGATACACGGAG | 5′-AAAGGGGTCACATCTACTCC |
| GST-pi | 5′-TTCAGTCCAACGCCATGC | 5′-ATGAGATCTGATCGCCAACC |
| Tocopherol transport protein | 5′-GTGTTTTGCTCATGCTCTGC | 5′-ACTTCATCTACGCTGGGTCC |
Statistical Analysis
All statistics were compiled using SigmaStat and plotted using Sigma Plot (SPSS Inc, Chicago, IL). Two-way analysis of variance (ANOVA) was used to detect significant differences between control and treated groups at a p < 0.05. All exposure studies had an N = 24 with 80% confidence interval. Significance was determined for cellular death assays using two-way ANOVA (p < 0.05), N = 12.
Results
Light activation of C60
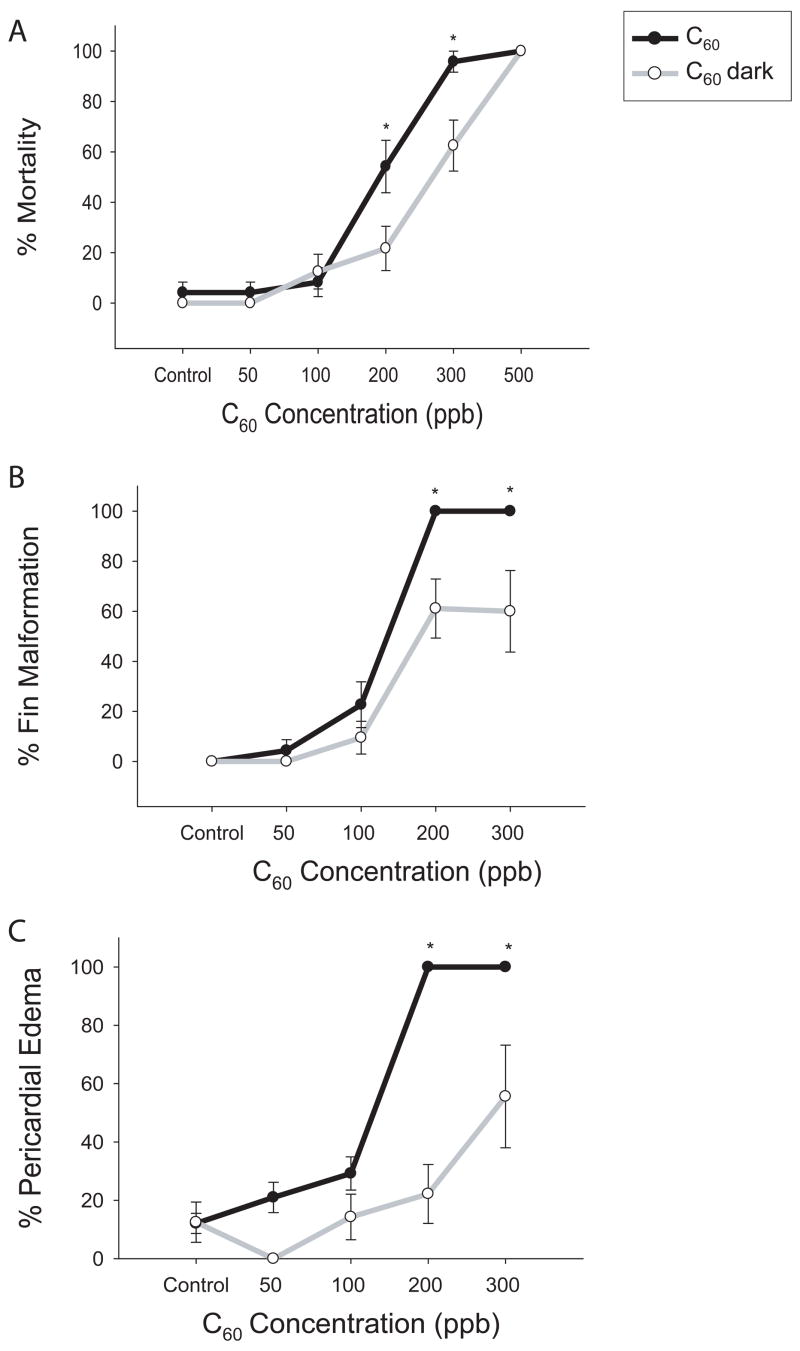
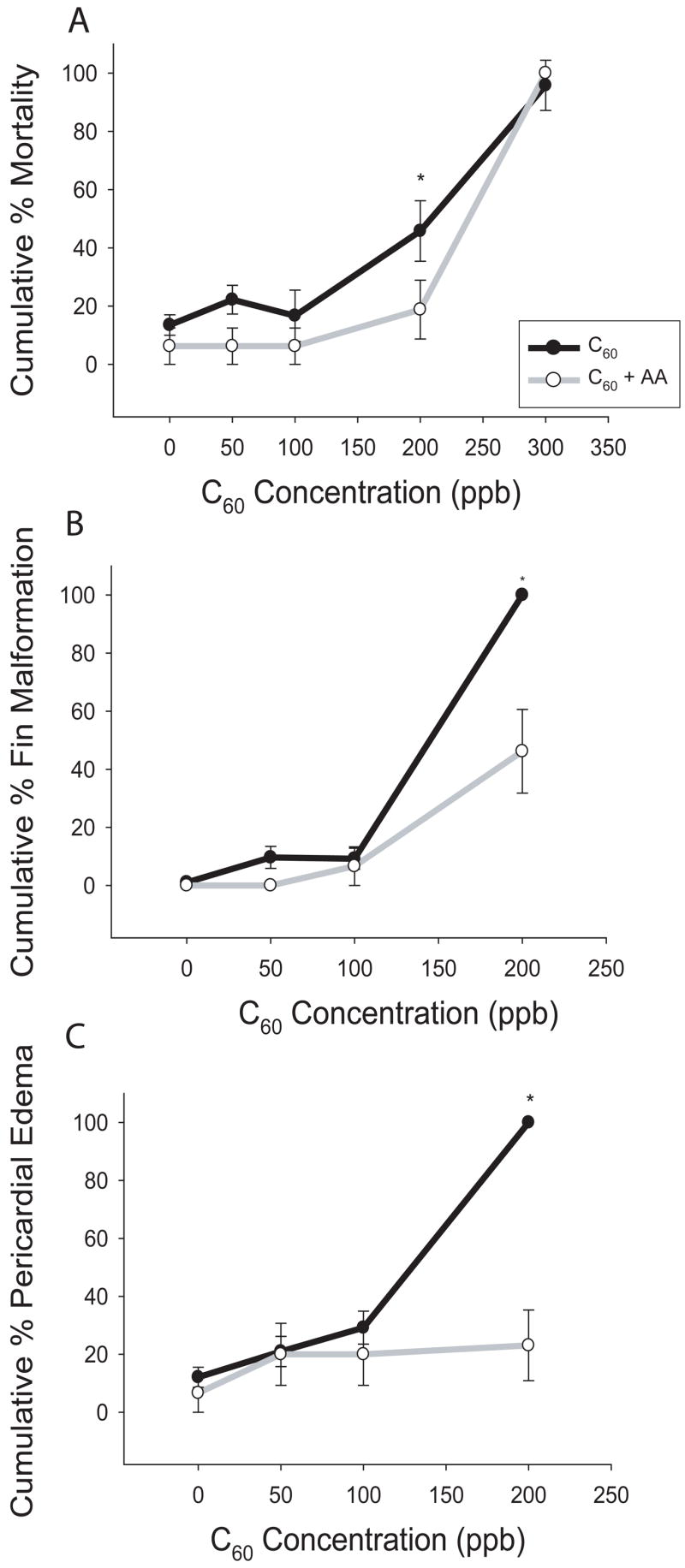
Oxidative stress was probed at the physical-chemical level since ROS generation by C60 has been shown to be photoactivated (Wang et al., 1999). Although exposure in the dark was not completely protective, it did reduce the physiologic response of C60 exposure. There was a significant reduction in fin malformations, pericardial edema and mortality in the 200 and 300 ppb exposure groups when exposed in the dark (figure 1). Mortality was reduced by 30% at 200 and 300 ppb; however, 500 ppb C60 still induced 100% mortality within the first 24 hours of exposure (figure 1 A). Fin malformations were reduced by approximately 40% and pericardial edema was reduced by 85% at 200 ppb (figure 1 B, C). The presence or absence of light did not have an effect on development of the embryos.
Figure 1. C60 concentration-response: dark vs. light exposure.
24 hpf embryos were dechorionated and exposed in the dark to graded concentrations of C60. Mortality (A), fin malformations (B), and pericardial edema (C) were scored daily for 5 days post exposure. Significant difference was determined using two-way ANOVA (*p < 0.05), N = 24, compared to C60 effect in the light at that concentration. Error bars represent the standard error of means (SEM).
Chemical supplementation of GSH and ascorbic acid
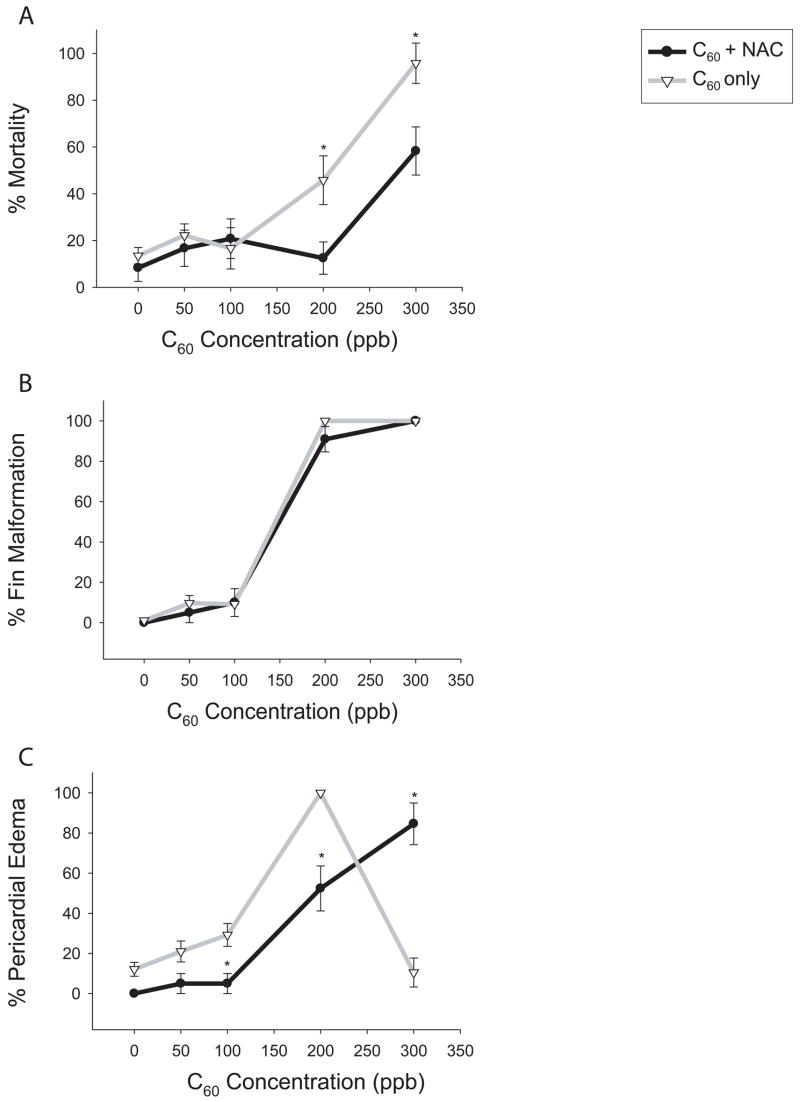
ROS-induced toxicity should be reduced by an increase in intracellular antioxidant levels. To determine if oxidative stress is a potential mechanism of C60-induced responses, chemical alterations in GSH levels were evaluated. Co-incubation of 100 μM GSH with a concentration gradient of C60 did not alter the responses elicited by C60 alone (data not shown). Due to the low membrane permeability of GSH, embryos were co-exposed to NAC rather than GSH to determine if favoring GSH synthesis would offer protection from C60-induced responses. NAC can induce toxicity at high concentrations >300 μM (data not shown) so a low concentration (50 μM) was co-incubated with a concentration gradient of C60 (50–300 ppb C60) (figure 2 A–C). NAC co-exposure with C60 reduced mortality by approximately 35% at 200 and 300 ppb and pericardial edema by 50% at 200 ppb C60 (figure 2 A,C). However, at concentrations of 300 ppb, the incidence of pericardial edema was significantly increased, most likely due to improved survival. NAC co-exposure to 1% DMSO did not have an effect compared to the 1% DMSO controls. Interestingly, co-exposure with NAC did not reduce the incidence of fin malformations at any concentration (figure 2 B).
Figure 2. NAC co-incubation with C60.
Embryonic zebrafish were co-incubated with 50 μM NAC and graded concentrations of C60 from 24 to 120 hpf. (A) Cumulative mortality by 120 hpf for C60 + NAC (●) and C60 only (▽). (B) Percentage of embryos with fin malformation including those that died prior to 120 hpf. (C) Percentage of embryos with pericardial edema including those later scored for mortality. Significance was determined using two-way ANOVA, (*p < 0.05, N = 24), and error bars represent the +/− the SEM.
An alternative approach to block ROS-induced toxicity is to increase intracellular antioxidant levels. Ascorbic acid (vitamin C) has been shown to protect zebrafish from ethanol-induced toxicity at 250 μM (Reimers et al., 2006). Embryos were co-exposed to 250 μM ascorbic acid and 50, 100, 200, and 300 ppb C60. At 200 ppb C60, there was a significant reduction in mortality; however at 300 ppb, 100% mortality was observed (figure 3 A). There was not a statistically significant difference between ascorbic acid controls (with 1% DMSO) and 1% DMSO controls. Unlike NAC, ascorbic acid reduced incidence of fin malformations at 200 ppb C60 (figure 3 B). At 200 ppb C60, there was nearly complete protection from pericardial edema compared to background levels (figure 3 C).
Figure 3. C60 co-incubation with ascorbic acid.
Embryos were co-exposed to 250 μM ascorbic acid (AA) and graded concentrations of C60 at 24 hpf until 120 hpf. Ascorbic acid decreased (A) mortality, (B) fin malformations (FM), and (C) pericardial edema (PE) at 200 ppb C60. Significance was determined by two-way ANOVA (*p < 0.05, N = 24), and error bars represent +/− the SEM.
Chemical depletion of GSH
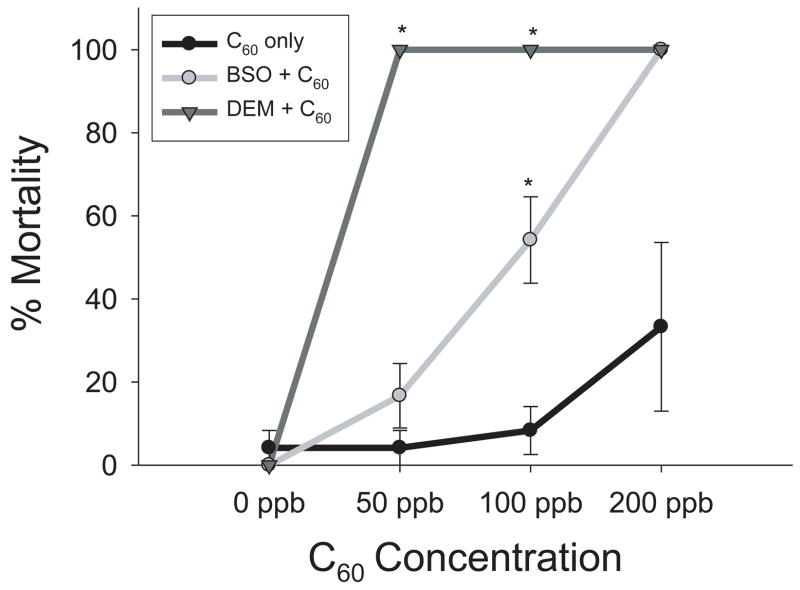
BSO and DEM, known inhibitors of glutathione synthesis, were used to determine if C60 exerts a toxic response through depletion of glutathione. DEM was found to have a maximum tolerable concentration (MTC) of 50 nM and BSO had a MTC of 5 μM. These concentrations of DEM and BSO were used for each co-incubation with graded concentrations of C60 (50–300 ppb) and 1% DMSO controls. Both DEM and BSO increased the embryo’s sensitivity to C60, with increased mortality within the first 24 hours of exposure (figure 4). Embryos were more sensitive to DEM than BSO. There was not a significant increase in mortality between controls for either DEM or BSO and control embryos.
Figure 4. DEM and BSO concentration response.
DEM and BSO were used to block glutathione production. Embryos were co-exposed to 5 μM BSO or 50 nM DEM and graded concentrations of C60 at 24 hpf for 24 hours. DEM shifted the LC50 to 50 ppb rather than 200 ppb. BSO induced 100% mortality at 200 ppb, but no significant mortality at 100 ppb. Significance was determined using two-way ANOVA (*p < 0.05, N = 24). Error bars represent +/− the SEM.
To more closely examine the C60-elicited physiologic response following exposure to NAC or DEM, cellular death was evaluated in whole embryos. Embryos were co-incubated with 100 ppb C60 and 50 nM DEM, 5 μM BSO, 250 μM ascorbic acid or 100 μM NAC. Control embryos were incubated with 1% DMSO, and chemical controls were also co-incubated with 1% DMSO. DEM, BSO, and NAC did not induce cellular death when co-incubated with 1% DMSO. However, DEM and BSO significantly increased cell death when co-exposed to C60 compared to C60 exposed embryos (figure 5). NAC exposure offered only partially protected the embryo from C60-induced cellular death. Ascorbic acid had no effect on cell death despite its protection from physiologic responses (data not shown).
Figure 5. Cell death in co-exposures.
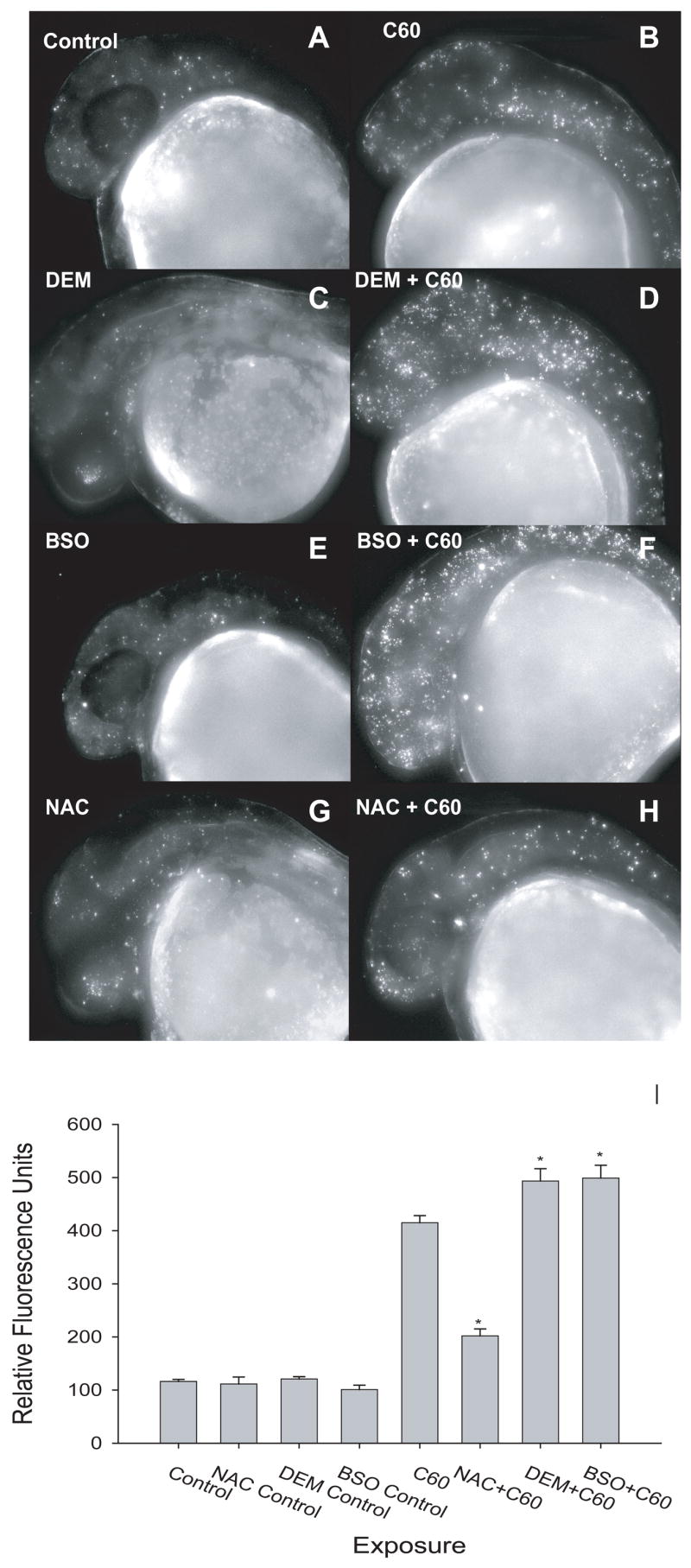
Cell death was measured as a result of co-incubation with either NAC or DEM and 100 ppb C60. Embryos were exposed at 24 hpf and cellular death was determined at 36 hpf using acridine orange. There was not a statistically significant difference between (A) control and (C) DEM, (E) BSO, or (G) NAC only. (D,E) DEM and BSO co-incubated with C60 significantly increased cell death compared to (B) C60 only. (H) NAC decreased cell death compared to C60 alone; however cell death was significantly higher than controls. Significance was determined using two-way ANOVA (*p < 0.05, N = 12).
Antioxidative Potential of Fullerenes
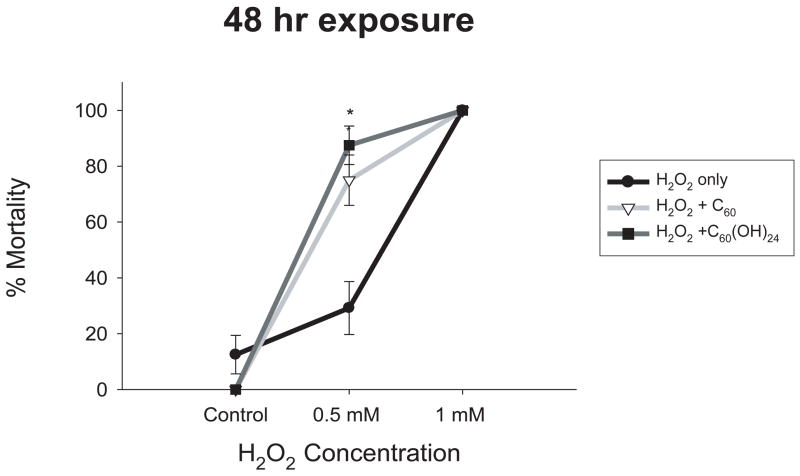
A study by Wang et al. (1999) found C60 to be a powerful antioxidant, even more powerful than vitamin E. So far, high levels of C60 have been investigated for oxidative potential; however, antioxidant properties may be observed only at very low concentrations. Low concentrations of C60 and C60(OH)24 (10 ppb and 500 ppb, respectively) were co-incubated with two concentrations of H2O2 (500 μM and 1 mM) to determine if C60 offered protection from H2O2-induced oxidative stress and mortality. These fullerene concentrations were previously reported to be 10-fold below the no observable adverse effect level (NOAEL) (Kamat et al., 1998; Kamat et al., 2000). Neither co-exposure offered embryonic protection, but instead significantly increased mortality compared to embryos exposed only to H2O2 (figure 6).
Figure 6. H2O2 and C60 co-incubation.
Embryos were exposed to H2O2 at 24 hpf for 48 hours to determine the concentration-response. Embryos were co-exposed to H2O2 and either 10 ppb C60 or 500 ppb C60(OH)24 for 48 hours. Both significantly increased mortality at 0.5 mM H2O2. Significance was determined using two-way ANOVA (*p < 0.05, N = 24). Error bars represent +/− the SEM.
Global Gene Expression
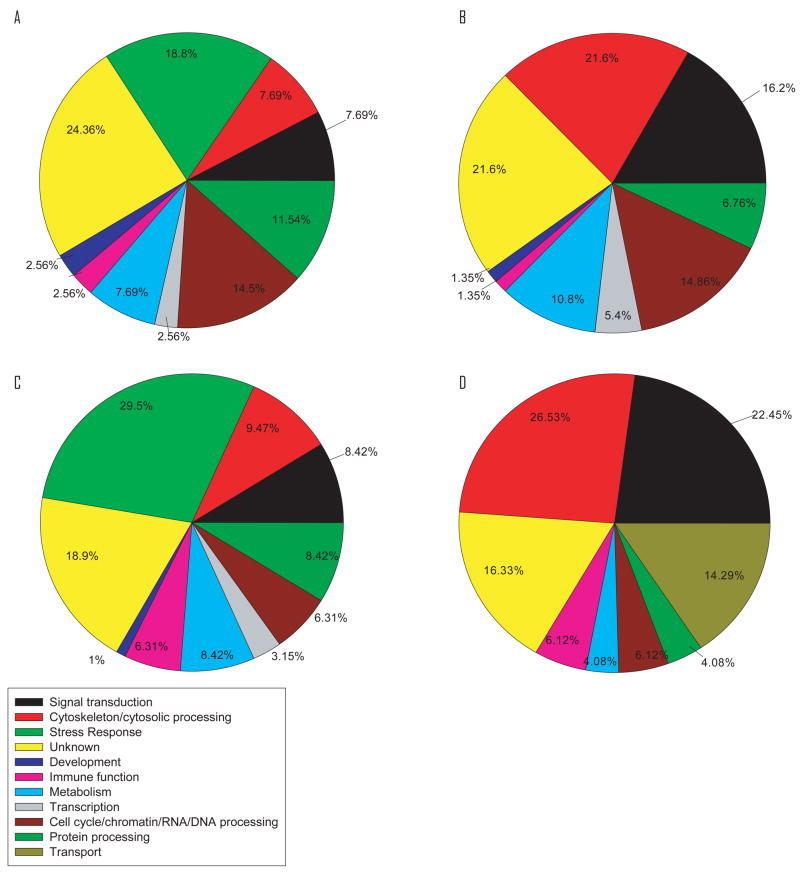
Custom zebrafish microarrays were used to identify the early genomic responses to C60 exposure. The microarray data was analyzed to determine the transcripts that were mis-regulated by more than 2 fold following exposure. Of the 234 and 95 genes that were up-regulated by C60 exposure at 36 hpf and 48 hpf, respectively, 66 genes were up-regulated at both time points. Only 15 genes were commonly down-regulated between the two time points. Genes were grouped by function and time of exposure (figure 7 A–D). Noteworthy, 24% and 19% of genes up-regulated at 36 and 48 hpf, respectively, were associated with a response to stress. These genes include GST-pi, GCLc, heat shock proteins and ferritin. Furthermore, there was up-regulation of development, cell cycle (including induction of apoptosis), signal transduction, and cytoskeleton and cytoplasmic transfer genes (figure 7). Examples of regulated genes from each category are given in table 2. See supporting information for complete list of genes. It is important to point out that for several of the genes the MWG oligo set contained multiple oligos for each target gene covering different regions of the transcript, and we found very little variation in intensity across array elements which further increased the confidence of the raw array data.
Figure 7. Pie chart of categories of gene regulation alterations.
Differentially regulated genes were put into broad categories by stage and regulation: (A) 36 hpf up, (B) 36 hpf down, (C) 48 hpf up, (D) 48 hpf down. Stress response genes were only found to be up-regulated at both stages.
Table 2.
Examples of misregulated genes. Several genes that were significantly up- or down-regulated at 36 hpf or 48 hpf compared to 1% DMSO controls were selected.
| Reporter ID | Gene Name | 36 hpf Average | 36Standard Deviation | 48 hpf Average | 48 hpf Standard Deviation |
|---|---|---|---|---|---|
| Cell cycle/chromatin/RNA/DNA processing | |||||
| mwgzebrafish#04109 | Biglycan-like protein 3 | 11.585 | 3.942 | 9.412 | 0.904 |
| mwgzebrafish#05567 | DCMP deaminase | 5.986 | 1.124 | 3.403 | 1.000 |
| mwgzebrafish#04079 | Mob1/phocein | 8.900 | 0.726 | 7.296 | 2.019 |
| mwgzebrafish#04110 | Wings apart | 5.195 | 1.279 | 4.688 | 2.163 |
| Cytoskeleton/Cytosolic Transport | |||||
| mwgzebrafish#05549 | E-cadherin binding protein E7 | 10.391 | 3.233 | 5.756 | 1.575 |
| mwgzebrafish#02873 | Keratin 18 | 3.726 | 0.862 | 3.134 | 0.539 |
| mwgzebrafish#06894 | Beta-actin | 0.280 | 0.023 | ||
| Immune Function | |||||
| mwgzebrafish#04096 | CTAGE family, member 5 | 4.665 | 0.864 | 3.875 | 0.448 |
| mwgzebrafish#04064 | Tec-family kinase | 4.956 | 0.705 | 3.487 | 1.024 |
| Metabolism | |||||
| mwgzebrafish#04095 | Angiotensin converting enzyme | 7.947 | 0.739 | 6.020 | 0.822 |
| mwgzebrafish#00919 | Cbr1l protein | 8.035 | 1.971 | 5.063 | 0.540 |
| mwgzebrafish#11641 | Inosine triphosphate pyrophosphatase | 5.625 | 3.587 | 2.715 | 0.156 |
| mwgzebrafish#13913 | Transketolase | 3.112 | 0.280 | 3.040 | 0.350 |
| Protein Processing/Degradation | |||||
| mwgzebrafish#12741 | Cathepsin B | 2.627 | 0.208 | 3.031 | 0.294 |
| mwgzebrafish#04081 | Esrom | 8.228 | 0.201 | 7.710 | 2.500 |
| mwgzebrafish#08710 | Ubiquitin C | 3.708 | 1.163 | 2.859 | 0.759 |
| Signal Transduction | |||||
| mwgzebrafish#07570 | CaM kinase | 18.887 | 4.605 | 6.425 | 1.833 |
| mwgzebrafish#01242 | Neurogenin 1 | 10.825 | 4.724 | 5.662 | 0.877 |
| mwgzebrafish#05534 | RNA-binding region RNP-1 | 8.312 | 0.999 | 5.003 | 0.656 |
| Stress Response | |||||
| mwgzebrafish#01262 | Appb protein | 6.920 | 0.908 | 5.730 | 0.667 |
| mwgzebrafish#11036 | Ferritin | 15.994 | 1.393 | 7.482 | 1.976 |
| mwgzebrafish#05998 | Ferritin subunit 1 | 18.070 | 1.657 | 9.912 | 1.624 |
| mwgzebrafish#00125 | Glutathione S-transferase pi | 11.527 | 1.276 | 9.723 | 0.781 |
| mwgzebrafish#13567 | Major vault protein | 2.865 | 0.421 | 2.977 | 0.711 |
| mwgzebrafish#08871 | Sulfotransferase | 4.616 | 0.953 | 3.931 | 1.624 |
| mwgzebrafish#03954 | Thioredoxin peroxidase | 13.581 | 1.584 | 5.747 | 0.680 |
| mwgzebrafish#00078 | Hsp70 | 6.991 | 1.046 | 2.984 | 0.515 |
| mwgzebrafish#07774 | Glutamate-cysteine ligase, catalytic subunit alpha-tocopherol | 4.926 | 0.677 | ||
| mwgzebrafish#05558 | transfer protein | 4.173 | 1.006 | ||
| mwgzebrafish#00120 | Ferritin heavy chain | 2.716 | 0.112 | 2.750 | 0.359 |
PCR
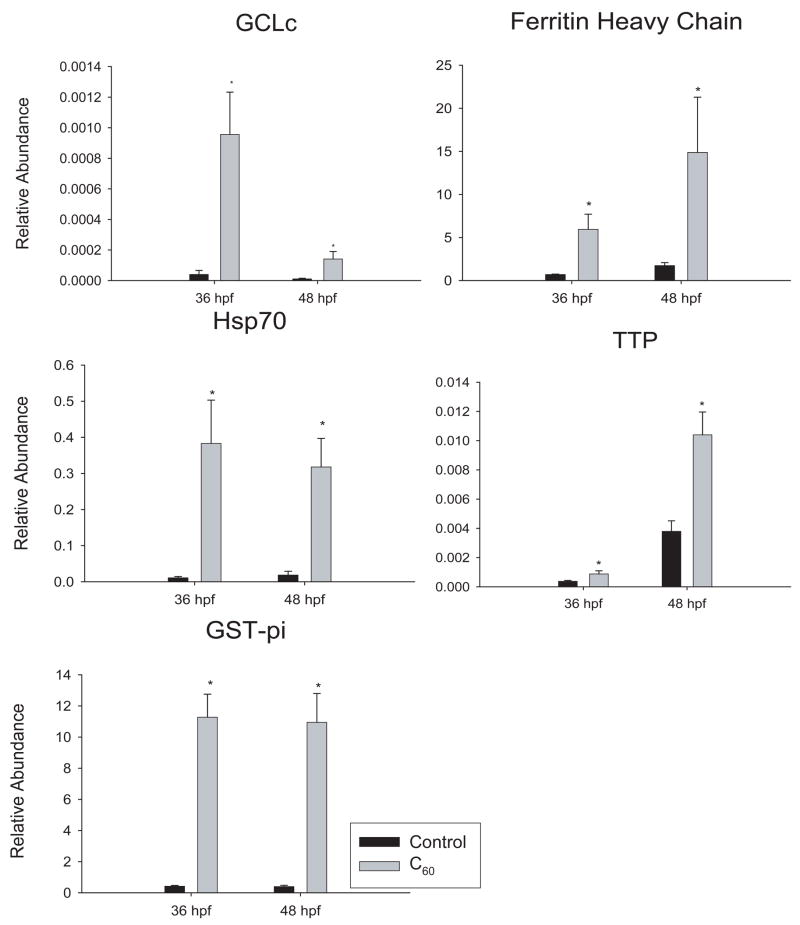
Five target genes that were found to be differentially expressed by microarray analysis were validated using qPCR: GST-pi, GCLc, ferritin heavy chain, α-tocopherol transport protein (TTP), and heat shock protein 70kD (HSP70). All genes chosen were significantly up-regulated over 2-fold. GAPDH was used as a loading control and adult zebrafish cDNA was used as a calibrator. These stress response genes were significantly up-regulated (figure 8). The abundance of GCLc was very low, resulting in a large fold induction. GST-pi had strong results with similar fold induction at both 36 and 48 hpf (figure 8 A). The results from PCR validated the directional regulation observed in the microarray analyses.
Figure 8. qRT-PCR results of gene expression.
Genes involved in oxidative stress responses that were identified as mis-regulated by the microarray were further validated using qRT-PCR. Significance was determined using one-way ANOVA between controls and 200 ppb C60 treated (*p < 0.05, N = 3). Error bars represent the SEM.
Discussion
In vivo evaluations conducted using embryonic zebrafish provide strong evidence indicating C60 is a powerful oxidant in the absence of functionalization. Four lines of evidence presented herein point to oxidative stress as a primary pathway of C60-induced toxicity. First, physiologic responses and cellular death were inhibited by the absence of light during solution preparation and throughout the exposure period. A reduction in light resulted in reduced mortality and malformations, except at the highest concentration. While photoactivation strongly indicates oxidative stress as a mechanism of action, the lack of complete protection when exposed in the dark indicates this may not be the only mechanism of action. Lee et al. (2007) recently found that agglomeration size of C60 affects the degree to which C60 is able to mediate energy and electron transfer (Lee et al., 2007). The large agglomeration sizes previously measured in our system indicate there could be a decrease in photoirradiation potential of C60 at higher concentrations (Kamat et al., 2000; Yamakoshi et al., 2003). Kamat and others demonstrated that when photoexcited, both C60 and hydroxylated C60 can induce membrane damage in rat hepatic and tumor microsomes by generation of ROS in a time- and concentration-dependent manner (Prat et al., 1999). Although these photoactivation characteristics may be undesirable for healthy cells, this activity could be exploited for applications in biomedical science (Zhu et al., 2007). For example, targeted drug delivery to tumors may be achieved via encapsulation of chemotherapeutics in C60 particles and subsequent illumination localized at the target site to activate C60 and trigger release of the drug. Additionally, surface groups could be used to alter the photochemical properties of fullerenes since they are apparently less responsive to light with increased functionalization (Kamat et al., 1998; Sayes et al., 2005).
A second line of evidence is provided by the change in sensitivity of embryonic zebrafish to C60 exposure that resulted from altered levels of the antioxidants GSH and ascorbic acid. Although administration of GSH itself did not have an effect on embryonic susceptibility, uptake of GSH into cells is thought to be limited requiring ATP for transport (Wang et al., 1999). However, another study using embryonic zebrafish revealed that GSH co-exposure with tetrahydrofuran-C60 (THF-C60) increased survival when compared to THF-C60 alone (Bogdanovic et al., 2004). In this case, it may be that solubilization of C60 with THF changed its physicochemical properties such that GSH interacted directly with THF-C60 in the exposure solution and reduced C60 uptake. Internal levels of GSH would need to be measured before and after waterborne GSH exposure to determine its uptake into biological systems.
Uptake limitations and uncertainties of GSH were overcome by using chemical that could deplete internal antioxidant pools. Depletion of antioxidants is one pathway through which nanomaterials could induce oxidative toxicity. When DEM and BSO were used to chemically inhibit glutathione production in embryonic zebrafish, the concentration-response curve shifted to the left; that is, a lower concentration of C60 was required to induce the same effects observed at higher concentrations. This not only implicates oxidative stress as a mechanism of C60 toxicity but also the highlights the importance of GSH in mediating the response. While DEM and BSO potentiated the effects of C60, the addition of NAC offered protection from C60-induced toxicity. NAC had been used in previous studies to increase GSH levels and protect organisms from oxidative stress (Kamat et al., 1998; Sayes et al., 2005). Similarly, our results demonstrated a significant reduction in mortality and pericardial edema in NAC co-incubated embryos. Fin malformations, however, were still present in all embryos exposed to concentrations of 200 ppb and 300 ppb. Co-exposure of embryos to C60 and ascorbic acid resulted in a significant reduction in mortality, pericardial edema and fin malformations, though at 300 ppb C60, vitamin C could no longer provide protection and 100% of the embryos died. Our results concur with previous studies that found ascorbic acid to protect against C60-induced cell death and lipid peroxidation. Collectively, these results suggest C60 has oxidative potential since depletion of antioxidants increased the sensitivity of zebrafish embryos to C60 exposure while antioxidant enhancement decreased their sensitivity.
The lack of antioxidative function observed from both C60 and C60(OH)24 when co-exposed with H2O2 provides the third line of evidence supporting an oxidative stress echanism of toxic action. Cell culture evaluations suggest that C60 acts as a stronger antioxidant than vitamin E, protecting against 1 mM H2O2. Derivatives of C60 have also been shown to exhibit antioxidant properties in numerous cell culture and whole animal studies (Sayes et al., 2004; Kamat et al., 1998; Yamakoshi et al., 2003; Sayes et al., 2005). Despite previous reports of antioxidant capabilities, neither C60 nor C60(OH)24 demonstrated antioxidant properties in the embryonic zebrafish model. In fact, rather than offering protection from H2O2 exposure, both C60 and its hydroxylated derivative significantly increased the deleterious oxidative effects. Delineation of those that possess antioxidant potential and those that do not should be addressed systematically since ‘fullerenes’ are already being marketed to consumers as antioxidants in cosmetics and night creams. Additionally, identification of fullerenes with antioxidant potential could be developed as therapeutic applications for central nervous system neurodegenerative diseases (i.e. Parkinson’s disease, Alzheimer’s disease, multiple sclerosis, amyotrophic lateral sclerosis, or Huntington’s disease), stroke, atherosclerosis, myocardial ischemia, myocardial reperfusion, diabetes, complications of diabetes, circulatory impairment, retinopathy, blindness, kidney disease, pancreas disease, neuropathy, gum disease, cataracts, skin disease, skin damage, radiation damage, damage caused by tobacco use, excessive angiogenesis, insufficient angiogenesis, hearing loss, collateral damage of chemotherapy, mucositis, senescence, hemorrhagic shock, distributive shock, septic shock, heat stroke, severe burn shock, or non-hemorrhagic trauma shock (Hartnagel et al., 2006).
A final line of evidence indicates an oxidative stress mechanism of C60 toxicity. A zebrafish microarray developed from commercially available oligonucleotides was used to determine important genes and pathways that play a role in the response to C60 exposure. By analyzing two early time points during development, we were able to identify impacts that persist over multiple stages of development. Many of the genes known to be involved in an oxidative stress response were upregulated in embryonic zebrafish after exposure to C60. In particular, two genes that were significantly up-regulated are directly related to glutathione activity: GST-pi and GCLc. GCL is important for increasing GSH levels during oxidative stress, while GST is involved in the phase II metabolism conjugation of GSH to electrophilic xenobiotics. A study by Henry et al. also found GST-pi to be up-regulated; however, they attributed the up-regulation to the THF vehicle (Henry et al., 2007). In our study, 1% DMSO was used as the vehicle, not THF, so the common alterations in gene expression were likely due to the C60 and not the solvent vehicle.
Microarray results revealed increased expression of additional oxidative stress-responsive genes. An increase in α-tocopherol transfer proteins (TTP) transcript levels further implicates an oxidative stress response and is in accordance with the previous studies which found α-tocopherol protected cells in culture from C60-induced injury (Kamat et al., 1998; Sayes et al., 2005). Ferritin is a critical protein in detoxifying oxidative stress that has been linked to an antioxidant response element (ARE) (Iwasaki et al., 2006). Up-regulation of ferritin in response to C60 exposure indicates a disruption in iron homeostasis and/or oxidative stress. Ferritin is regulated by levels of iron, cytokines, hormones and oxidative stress (Zandman-Goddard and Shoenfeld, 2007). There is a chance that C60 had direct effects on these factors and subsequent indirect effects on ferritin transcription; however, it is more likely that ferritin was up-regulated to bind iron and prevent propagation of the oxidative stress cascade. The role of iron and ferritin for oxidative stress responses is an important area of research, particularly with regards to their interbalance following a stroke and in neurodegenerative disorders such as Alzheimer’s disease (Quitana et al., 2006).
Disparity among reports on C60 oxidative potential could be attributed to the wide range of methods for solution preparation which likely have an impact on the biological activity and toxic potential of fullerenes. Our experimental design called for sonication of C60 in 100% DMSO with dilution to final exposure concentrations of 1% DMSO. Granted, the use of such a solvent would increase potential uptake but we were interested in specifically detailing the interactions of fullerenes within biological systems, not assessing uptake from the environment into the system. Other methods of solubilization include the use of THF, ethanol, methanol or stirring for prolonged periods in direct sunlight (Yamago et al., 1995; Oberdorster, 2004; Sayes et al., 2004; Dhawan et al., 2006; Levi et al., 2006; Lovern and Klaper, 2006; Zhu et al., 2006). It is important to recognize that extensive methods of solubilization may also alter the physicochemical properties of C60, thereby altering the interaction within the biological system. For example, with increased hydroxylation, the photosensitivity of C60 decreases. This could be of potential concern for protocols that require prolonged stirring of C60 in direct sunlight.
Results presented herein are in agreement with the studies that show C60 can act as a pro-oxidant and elicit a toxic response via oxidative stress (Kamat et al., 1998; Yamakoshi et al., 2003; Oberdorster, 2004; Sayes et al., 2005). Chemically increasing antioxidant levels in vivo reduced adverse effects, while chemically inhibiting glutathione production increased sensitivity to C60 exposure. Functionalization of C60 may alter the biological response; however, there was no indication in this study to suggest that hydroxylation of C60 imparts antioxidant properties. Future studies should be focused on the role surface functionalization and methods of C60 preparation have on oxidative potential. The embryonic zebrafish has once again proven to be a valuable model for studying nanomaterial-biological interactions at multiple levels of biological organization, i.e. whole animal, cellular and molecular.
Acknowledgments
The authors would like to thank Eric Johnson and Jason Carriere for their assistance with the microarray slide preparation and hybridization protocol. We would also like to thank Caprice Rosato the Center for Gene Research and Biocomputing and Abby Benninghoff for assistance with microarray hybridization and data analysis. We would also like to acknowledge Jane LaDu for technical assistance. These studies were partially supported by the Oregon State University Research Office, the Safer Nanomaterials and Nanomanufacturing Initiative of the Oregon Nanoscience and Microtechnologies Institute, EPA STAR grant RD-833320 and NIEHS grants ES03850 and ES07060.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson ME. Glutathione: an overview of biosynthesis and modulation. Chem Biol Interact. 1998;111–112:1–14. doi: 10.1016/s0009-2797(97)00146-4. [DOI] [PubMed] [Google Scholar]
- Asikainen TM, Huang TT, Taskinen E, Levonen AL, Carlson E, Lapatto R, Epstein CJ, Raivio KO. Increased sensitivity of homozygous Sod2 mutant mice to oxygen toxicity. Free Radic Biol Med. 2002;32:175–186. doi: 10.1016/s0891-5849(01)00776-6. [DOI] [PubMed] [Google Scholar]
- Bogdanovic G, Kojic V, Dordevic A, Canadanovic-Brunet J, Vojinovic-Miloradov M, Baltic VV. Modulating activity of fullerol C-60(OH)(22) on doxorubicin-induced cytotoxicity. Toxicol in Vitro. 2004;18:629–637. doi: 10.1016/j.tiv.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Dhawan A, Taurozzi JS, Pandey AK, Shan WQ, Miller SM, Hashsham SA, Tarabara VV. Stable colloidal dispersions of C-60 fullerenes in water: Evidence for genotoxicity. Environmental Science & Technology. 2006;40:7394–7401. doi: 10.1021/es0609708. [DOI] [PubMed] [Google Scholar]
- Dugan LL, Lovett E, Quick K, Hardt J. Carboxylated C60 increases metazoan lifespan (United States) 2003 [Google Scholar]
- Dugan LL, Gabrielesen JK, Yu SP, Lin TS, Choi DW. Buckminsterfullerenol free radical scavengers reduce excitotoxic and apoptotic death of cultured cortical neurons. Neurobiology of Disease. 1996;3:129–135. doi: 10.1006/nbdi.1996.0013. [DOI] [PubMed] [Google Scholar]
- Dugan LL, Lovett EG, Quick KL, Lotharius J, Lin TT, O’Malley KL. Fullerene-based antioxidants and neurodegenerative disorders. Parkinsonism Relat D. 2001;7:243–246. doi: 10.1016/s1353-8020(00)00064-x. [DOI] [PubMed] [Google Scholar]
- Franklin CC, Krejsa CM, Pierce RH, White CC, Fausto N, Kavanagh TJ. Caspase-3-dependent cleavage of the glutamate-L-cysteine ligase catalytic subunit during apoptotic cell death. American Journal of Pathology. 2002;160:1887–1894. doi: 10.1016/S0002-9440(10)61135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharbi N, Pressac M, Hadchouel M, Szwarc H, Wilson SR, Moussa F. [60]fullerene is a powerful antioxidant in vivo with no acute or subacute toxicity. Nano Lett. 2005;5:2578–2585. doi: 10.1021/nl051866b. [DOI] [PubMed] [Google Scholar]
- Hartnagel U, Erlangen D, Hirsch A, Lebovitz R. fullerene compositions and their use as antioxidants (United States) 2006 [Google Scholar]
- Henry TB, Menn FM, Fleming JT, Wilgus J, Compton RN, Sayler GS. Attributing effects of aqueous C60 nano-aggregates to tetrahydrofuran decomposition products in larval zebrafish by assessment of gene expression. Environ Health Perspect. 2007 doi: 10.1289/ehp.9757. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch A, Brettreich M. Fullerenes: chemistry and reactions. Weinheim: Wiley-VCH; 2005. [Google Scholar]
- Isaacson CW, Usenko CY, Tanguay RL, Field JA. Quantification of fullerenes by LC/ESI-MS and its application to in vivo toxicity assays. Anal Chem. 2007;79:9091–9097. doi: 10.1021/ac0712289. [DOI] [PubMed] [Google Scholar]
- Isakovic A, Markovic Z, Todorovic-Markovic B, Nikolic N, Vranjes-Djuric S, Mirkovic M, Dramicanin M, Harhaji L, Raicevic N, Nikolic Z, Trajkovic V. Distinct cytotoxic mechanisms of pristine versus hydroxylated fullerene. Toxicological Sciences. 2006;91:173–183. doi: 10.1093/toxsci/kfj127. [DOI] [PubMed] [Google Scholar]
- Iwasaki K, Mackenzie EL, Hailemariam K, Sakamoto K, Tsuji Y. Hemin-mediated regulation of an antioxidant-responsive element of the human ferritin H gene and role of Ref-1 during erythroid differentiation of K562 cells. Mol Cell Biol. 2006;26:2845–2856. doi: 10.1128/MCB.26.7.2845-2856.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen AW, Wilson SR, Schuster DI. Biological applications of fullerenes. Bioorgan Med Chem. 1996;4:767–779. doi: 10.1016/0968-0896(96)00081-8. [DOI] [PubMed] [Google Scholar]
- Kamat JP, Devasagayam TPA, Priyadarsini KI, Mohan H. Reactive oxygen species mediated membrane damage induced by fullerene derivatives and its possible biological implications. Toxicology. 2000;155:55–61. doi: 10.1016/s0300-483x(00)00277-8. [DOI] [PubMed] [Google Scholar]
- Kamat JP, Devasagayam TPA, Priyadarsini KI, Mohan H, Mittal JP. Oxidative damage induced by the fullerene C-60 on photosensitization in rat liver microsomes. Chem-Biol Interact. 1998;114:145–159. doi: 10.1016/s0009-2797(98)00047-7. [DOI] [PubMed] [Google Scholar]
- Kimmel C, Ballard W, Kimmel S, Ullmann B, Schilling T. Stages of embryonic development of the zebrafish. Developmental Dynamics. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Lee J, Fortner JD, Hughes JB, Kim JH. Photochemical production of reactive oxygen species by C60 in the aqueous phase duirng UV irradiation. Environmental Science & Technology. 2007;41:2529–2535. doi: 10.1021/es062066l. [DOI] [PubMed] [Google Scholar]
- Levi N, Hantgan RR, Lively MO, Carroll DL, Prasad GL. C60-Fullerenes: detection of intracellular photoluminescence and lack of cytotoxic effects. Journal of Nanobiotechnology. 2006;4:14–25. doi: 10.1186/1477-3155-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loutfy RO, Lowe TP, Moravsky AP, Katagiri S. Perspectives of fullerene nanotechnology. Netherlands: Springer; 2002. Commercial production of fullerenes and carbon nanotubes; pp. 35–46. [Google Scholar]
- Lovern SB, Klaper R. Daphnia magna mortality when exposed to titanium dioxide and fullerene (C60) nanoparticles. Environmental Toxicology & Chemistry. 2006;25:1132–1137. doi: 10.1897/05-278r.1. [DOI] [PubMed] [Google Scholar]
- Mori T, Takada H, Ito S, Matsubayashi K, Miwa N, Sawaguchi T. Preclinical studies on safety of fullerene upon acute oral administration and evaluation for no mutagenesis. Toxicology. 2006;225:48–54. doi: 10.1016/j.tox.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Oberdorster E. Manufactured nanomaterials (fullerenes, C60) induce oxidative stress in the brain of juvenile largemouth bass. Environmental Health Prospectives. 2004;112:1058–1062. doi: 10.1289/ehp.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering KD, Wiesner MR. Fullerol-sensitized production of reactive oxygen species in aqueous solution. Environmental Science & Technology. 2005;39:1359–1365. doi: 10.1021/es048940x. [DOI] [PubMed] [Google Scholar]
- Prat F, Stackow R, Bernstein R, Qian WY, Rubin Y, Foote CS. Triplet-state properties and singlet oxygen generation in a homologous series of functionalized fullerene derivatives. J Phys Chem A. 1999;103:7230–7235. [Google Scholar]
- Quitana C, Bellefqih S, Laval JY, Guerquin-Kern JL, Wu TD, Avila J, Ferrer I, Arranz R, Patino C. Study of the localization of iron, ferritin, and hemosiderin in Alzheimer’s disease hippocampus by analytical microscopy at the subcellular level. J Struct Biol. 2006:153. doi: 10.1016/j.jsb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Reimers MJ, La Du JK, Periera CB, Giovanini J, Tanguay RL. Ethanol-dependent toxicity in zebrafish is partially attenuated by antioxidants. Neurotoxicol Teratol. 2006;28:497–508. doi: 10.1016/j.ntt.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Robichaud CL, Tanzil D, Weilenmann U, Wiesner MR. Relative risk analysis of several manufactured nanomaterials: an insurance industry context. Environ Sci Technol. 2005;39:8985–8994. doi: 10.1021/es0506509. [DOI] [PubMed] [Google Scholar]
- Sayes CM, Gobin AM, Ausman KD, Mendez J, West JL, Colvin VL. Nano-C60 cytotoxicity is due to lipid peroxidation. Biomaterials. 2005;26:7587–7595. doi: 10.1016/j.biomaterials.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Sayes CM, Fortner JD, Guo W, Lyon D, Boyd AM, Ausman KD, Tao Y, Sitharaman B, Wilson L, Hughes J, West J, Colvin V. The differential cytotoxicity of water-soluble fullerenes. Nano Lett. 2004;4:1881–1887. [Google Scholar]
- Suh JH, Shenvi SV, Dixon BM, Liu H, Jaiswal AK, Liu RM, Hagen TM. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci U S A. 2004;101:3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usenko CY, Harper SL, Tanguay RL. In vivo evaluation of carbon fullerene toxicity using embryonic zebrafish. Carbon. 2007 doi: 10.1016/j.carbon.2007.04.021. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang IC, Tai LA, Lee DD, Kanakamma PP, Shen CKF, Luh TY, Cheng CH, Hwang KC. C-60 and water-soluble fullerene derivatives as antioxidants against radical-initiated lipid peroxidation. Journal of Medicinal Chemistry. 1999;42:4614–4620. doi: 10.1021/jm990144s. [DOI] [PubMed] [Google Scholar]
- Wassenberg DM, Di Giulio RT. Synergistic embryotoxicity of polycyclic aromatic hydrocarbon aryl hydrocarbon receptor Agonists with cytochrome P4501A inhibitors in Fundulus heteroclitus. Environ Health Persp. 2004;112:1658–1664. doi: 10.1289/ehp.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamago S, Tokuyama H, Nakamura E, Kikuchi K, Kananishi S, Sioelo L, Nakahara J, Enomoto S, Ambe F. In vivo biological behavior of a water-miscible fullerene: 14C lableing, absorption, distribution, excretion and acute toxicity. Chemical Biology. 1995;2:385–389. doi: 10.1016/1074-5521(95)90219-8. [DOI] [PubMed] [Google Scholar]
- Yamakoshi Y, Umezawa N, Ryu A, Arakane K, Miyata N, Goda Y, Masumizu T, Nagano T. Active oxygen species generated from photoexcited fullerene (C60) as potential medicines: O2-* versus 1O2. J Am Chem Soc. 2003;125:12803–12809. doi: 10.1021/ja0355574. [DOI] [PubMed] [Google Scholar]
- Zandman-Goddard G, Shoenfeld Y. Ferritin in autoimmune diseases. Autoimmune Rev. 2007;6:457–463. doi: 10.1016/j.autrev.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Zhu S, Oberdorster E, Haasch ML. Toxicity of an engineered nanoparticle (fullerene, C60) in two aquatic species, Daphnia and fathead minnow. Mar Environ Res. 2006;62(Suppl):S5–9. doi: 10.1016/j.marenvres.2006.04.059. [DOI] [PubMed] [Google Scholar]
- Zhu X, Zhu L, Li Y, Duan Z, Chen W, Alvarez PJJ. Developmental toxicity in zebrafish (Danio Rerio) embryos after exposure to manufactured nanomaterials: buckminsterfullerene aggregates (nC60) and fullerol. Environmental and Toxicological Chemistry. 2007;26:976–979. doi: 10.1897/06-583.1. [DOI] [PubMed] [Google Scholar]