Abstract
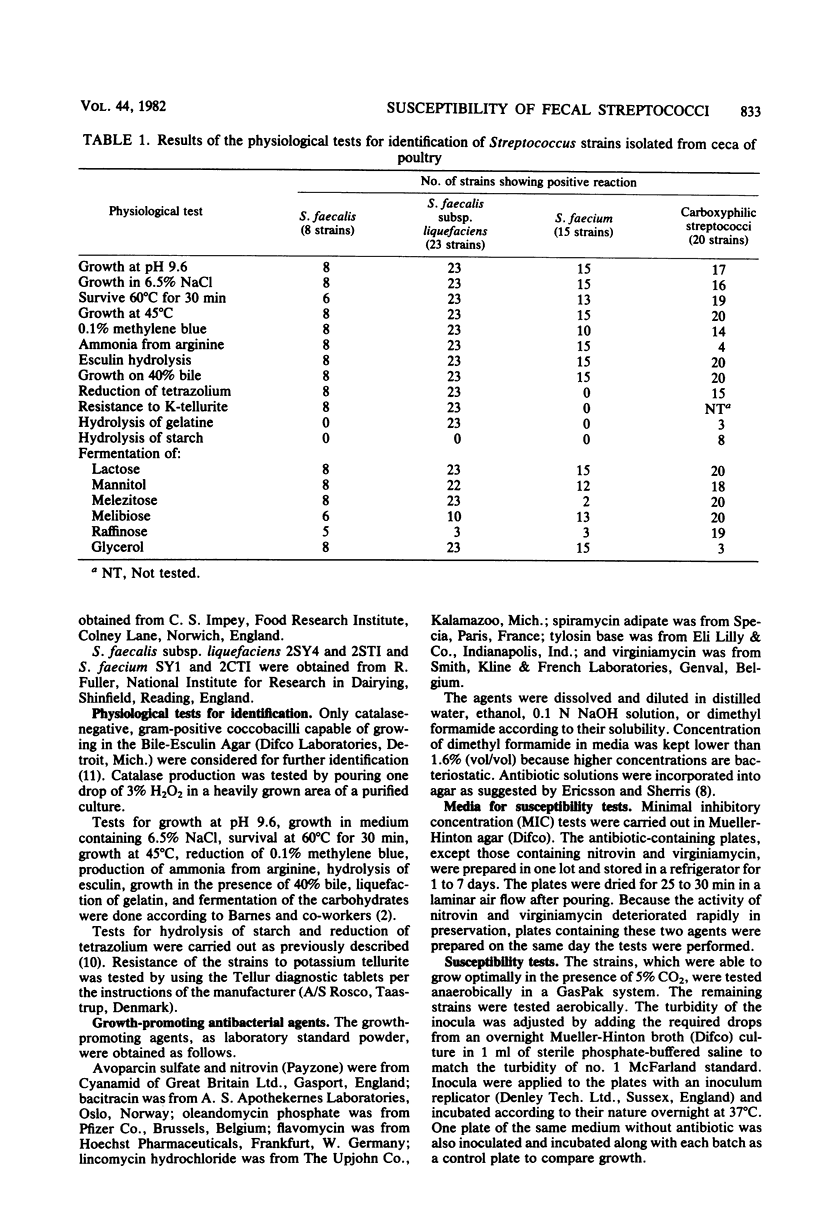
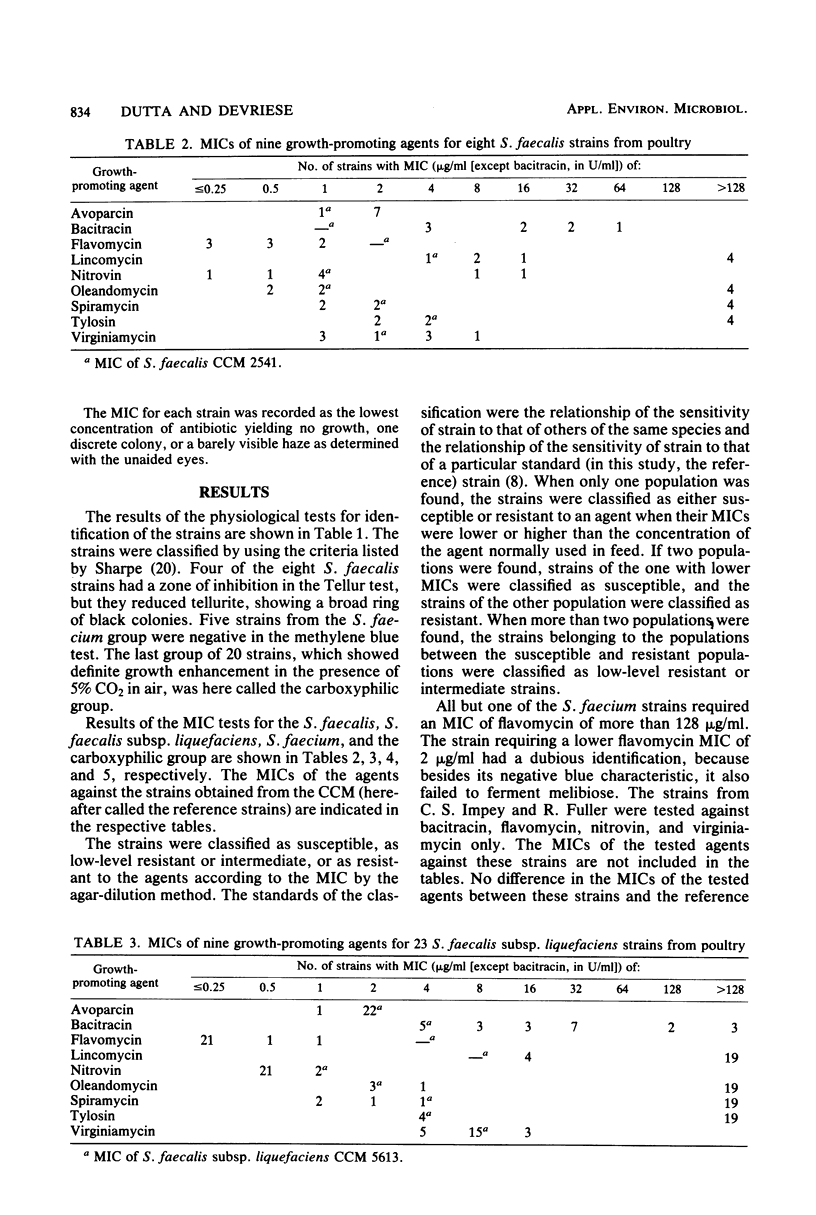
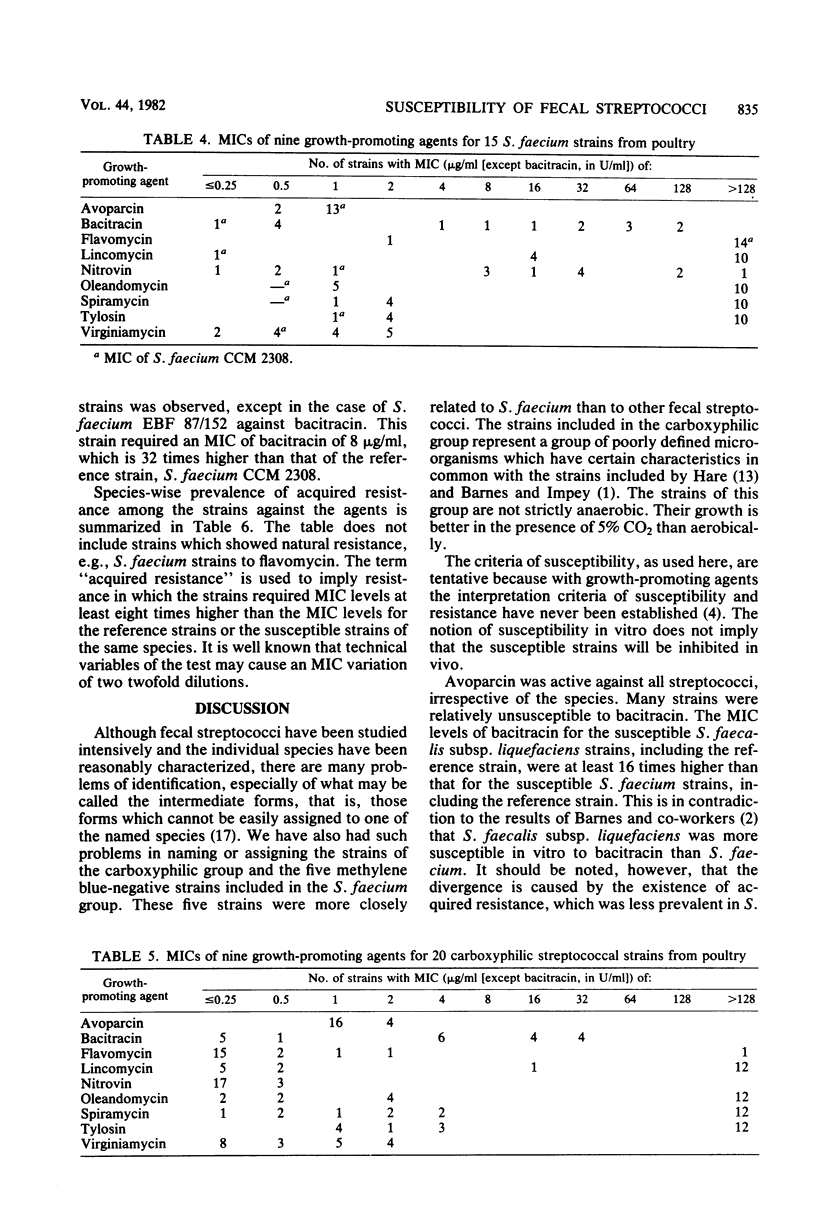
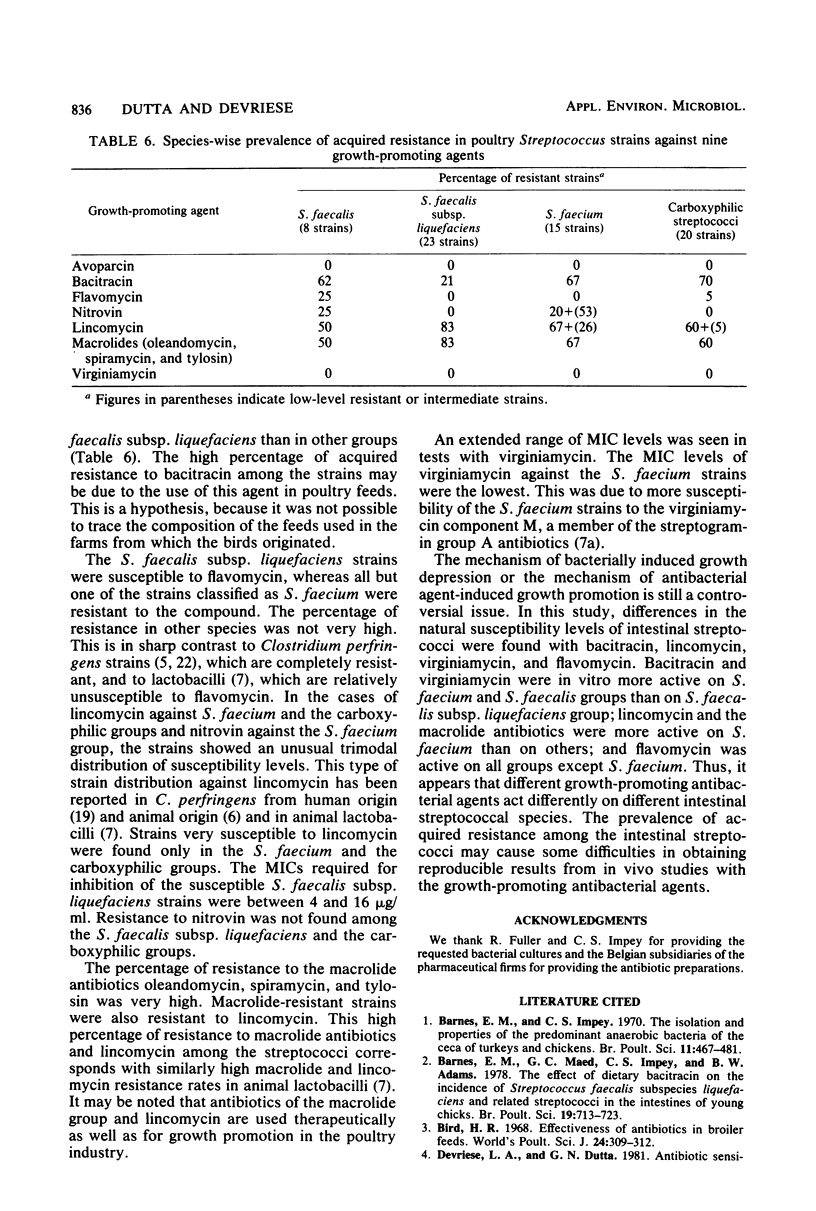
The minimal inhibitory concentrations of nine growth-promoting agents were determined by an agar-dilution method against 66 bile-tolerant streptococcal (8 Streptococcus faecalis, 23 Streptococcus faecalis subsp. liquefaciens, 15 Streptococcus faecium, and 20 carboxyphilic streptococci) strains isolated from the ceca of 52 chickens on 19 farms. Avoparcin was equally active on all groups. The natural susceptibilities against the other substances differed among the groups studied. Bacitracin and virginiamycin were more active on S. faecium and S. faecalis than on S. faecalis subsp. liquefaciens; lincomycin and the macrolide antibiotics were more active on S. faecium than on the other groups; and flavomycin was active on all groups except S. faecium. High percentages of acquired resistance were noted in all groups against bacitracin, lincomycin, and the macrolide antibiotics, oleandomycin, spiramycin, and tylosin. Resistance to nitrovin was found only among the S. faecalis and S. faecium groups.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes E. M., Impey C. S. The isolation and properties of the predominant anaerobic bacteria in the caeca of chickens and turkeys. Br Poult Sci. 1970 Oct;11(4):467–481. doi: 10.1080/00071667008415842. [DOI] [PubMed] [Google Scholar]
- Bird H. R. Effectiveness of antibiotics in broiler feeds. Worlds Poult Sci J. 1968 Oct-Dec;24(4):309–312. doi: 10.1079/wps19680030. [DOI] [PubMed] [Google Scholar]
- Devriese L. A., Dutta G. N. Antibiotic sensitivity testing: correlations between in vitro tests in vivo situations. Ann Rech Vet. 1981;12(1):41–46. [PubMed] [Google Scholar]
- Dutta G. N., Devriese L. A. Macrolide-lincosamide-streptogramin resistance patterns in Clostridium perfringens from animals. Antimicrob Agents Chemother. 1981 Feb;19(2):274–278. doi: 10.1128/aac.19.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta G. N., Devriese L. A. Resistance to macrolide-lincosamide-streptogramin antibiotics in enterococci from the intestines of animals. Res Vet Sci. 1982 Jul;33(1):70–72. [PubMed] [Google Scholar]
- Dutta G. N., Devriese L. A. Sensitivity and resistance to growth promoting agents in animal lactobacilli. J Appl Bacteriol. 1981 Oct;51(2):283–288. doi: 10.1111/j.1365-2672.1981.tb01243.x. [DOI] [PubMed] [Google Scholar]
- Ericsson H. M., Sherris J. C. Antibiotic sensitivity testing. Report of an international collaborative study. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;217(Suppl):1+–1+. [PubMed] [Google Scholar]
- Facklam R. R., Moody M. D. Presumptive identification of group D streptococci: the bile-esculin test. Appl Microbiol. 1970 Aug;20(2):245–250. doi: 10.1128/am.20.2.245-250.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facklam R. R. Recognition of group D streptococcal species of human origin by biochemical and physiological tests. Appl Microbiol. 1972 Jun;23(6):1131–1139. doi: 10.1128/am.23.6.1131-1139.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUHTANEN C. N., PENSACK J. M. THE ROLE OF STREPTOCOCCUS FAECALIS IN THE ANTIBIOTIC GROWTH EFFECT IN CHICKENS. Poult Sci. 1965 May;44:830–834. doi: 10.3382/ps.0440830. [DOI] [PubMed] [Google Scholar]
- Houghton S. B., Fuller R., Coates M. E. Correlation of growth depression of chicks with the presence of Streptococcus faecium in the gut. J Appl Bacteriol. 1981 Aug;51(1):113–120. doi: 10.1111/j.1365-2672.1981.tb00914.x. [DOI] [PubMed] [Google Scholar]
- Islam A. K. Rapid recognition of group-B Streptococci. Lancet. 1977 Jan 29;1(8005):256–257. doi: 10.1016/s0140-6736(77)91055-8. [DOI] [PubMed] [Google Scholar]
- Jones D. Composition and differentiation of the genus Streptococcus. Soc Appl Bacteriol Symp Ser. 1978;7:1–49. [PubMed] [Google Scholar]
- Ochi Y., Mitsuoka T., Sega T. Untersuchungen über die Darmflora des Huhnes. III. Die Entwicklung der Darmflora von Küken bis zum Huhn. Zentralbl Bakteriol Orig. 1964 Jun;193(1):80–95. [PubMed] [Google Scholar]
- Sapico F. L., Kwok Y. Y., Sutter V. L., Finegold S. M. Standardized antimicrobial disc susceptibility testing of anaerobic bacteria: in vitro susceptibility of Clostridium perfringens to nine antibiotics. Antimicrob Agents Chemother. 1972 Oct;2(4):320–325. doi: 10.1128/aac.2.4.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W. The development of the flora of the alimentary tract in young animals. J Pathol Bacteriol. 1965 Oct;90(2):495–513. [PubMed] [Google Scholar]


