Abstract
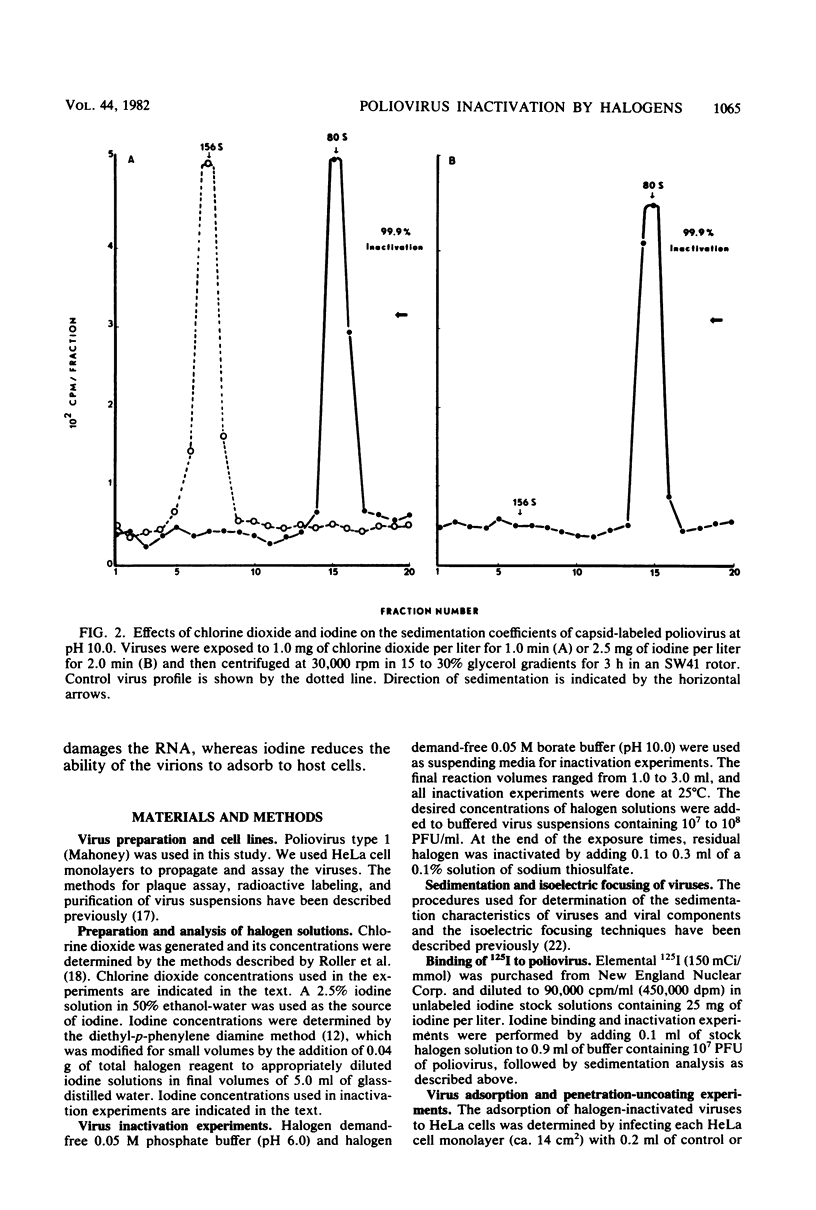
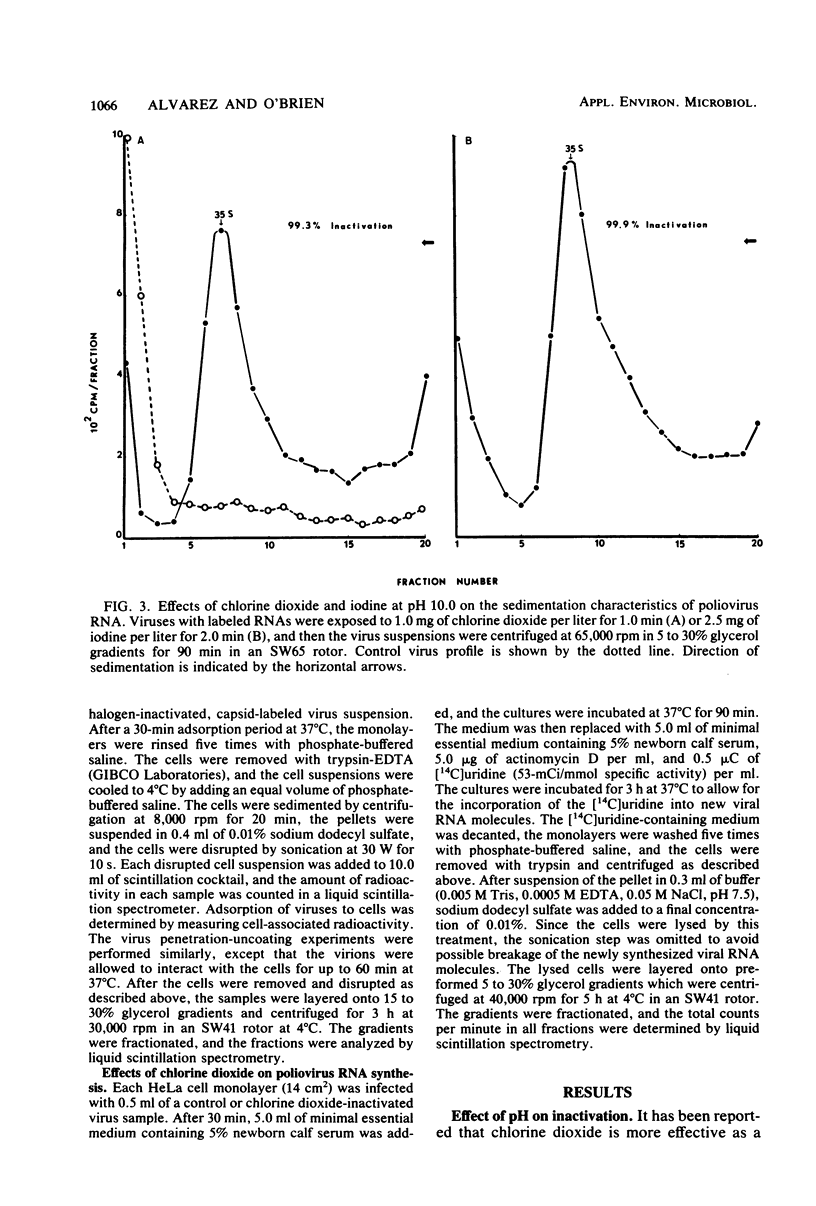
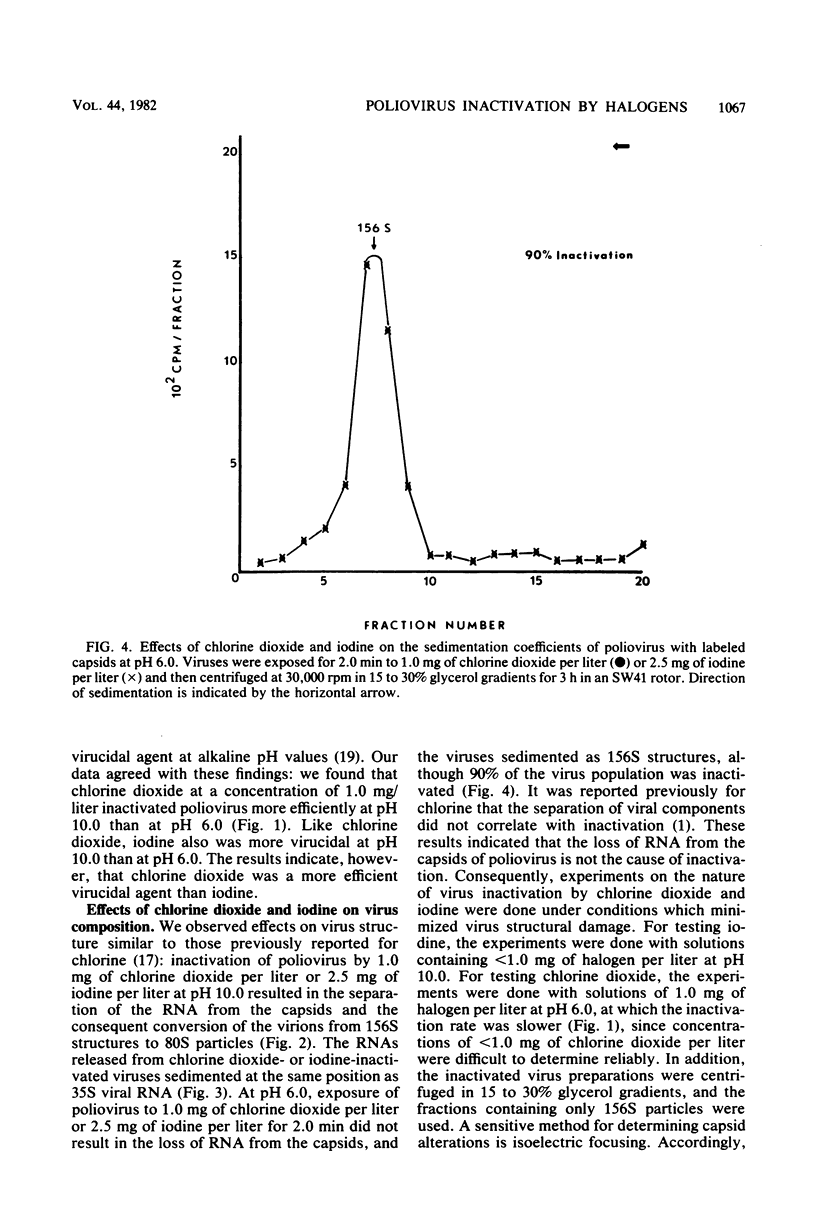
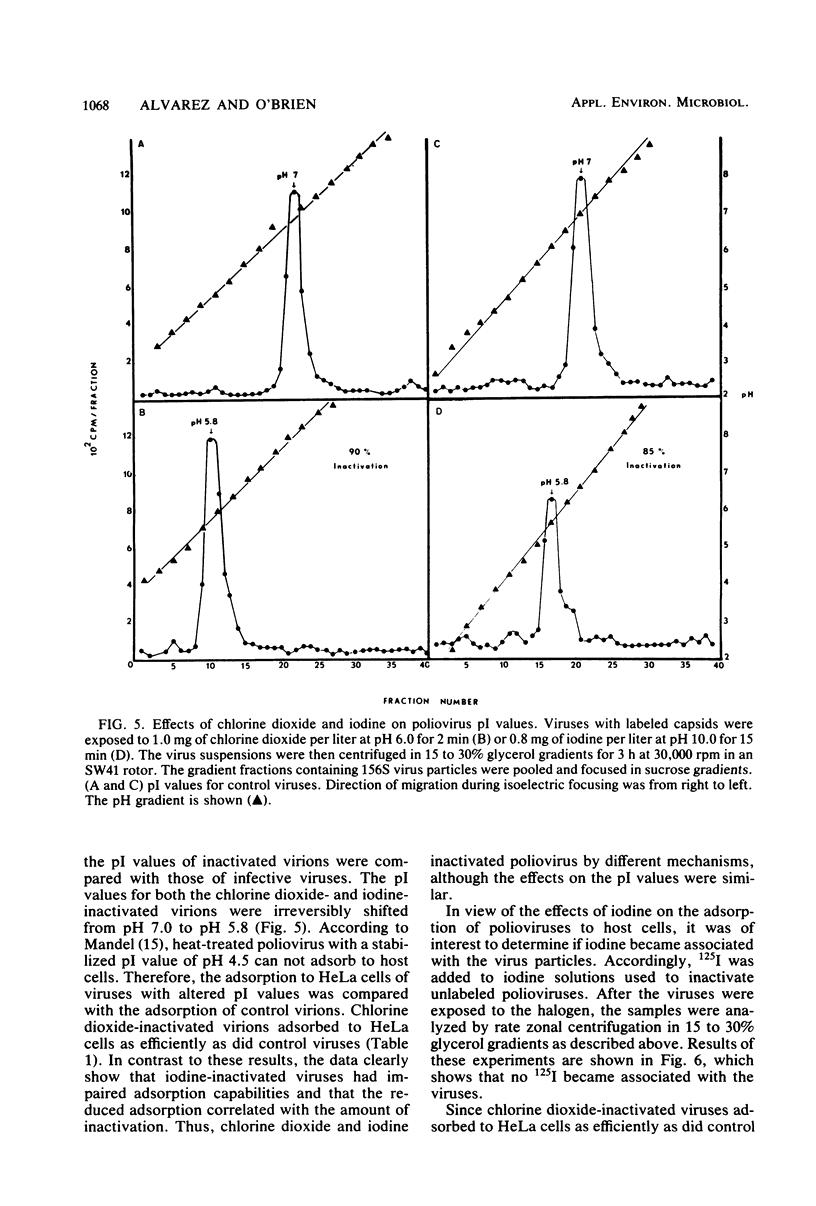
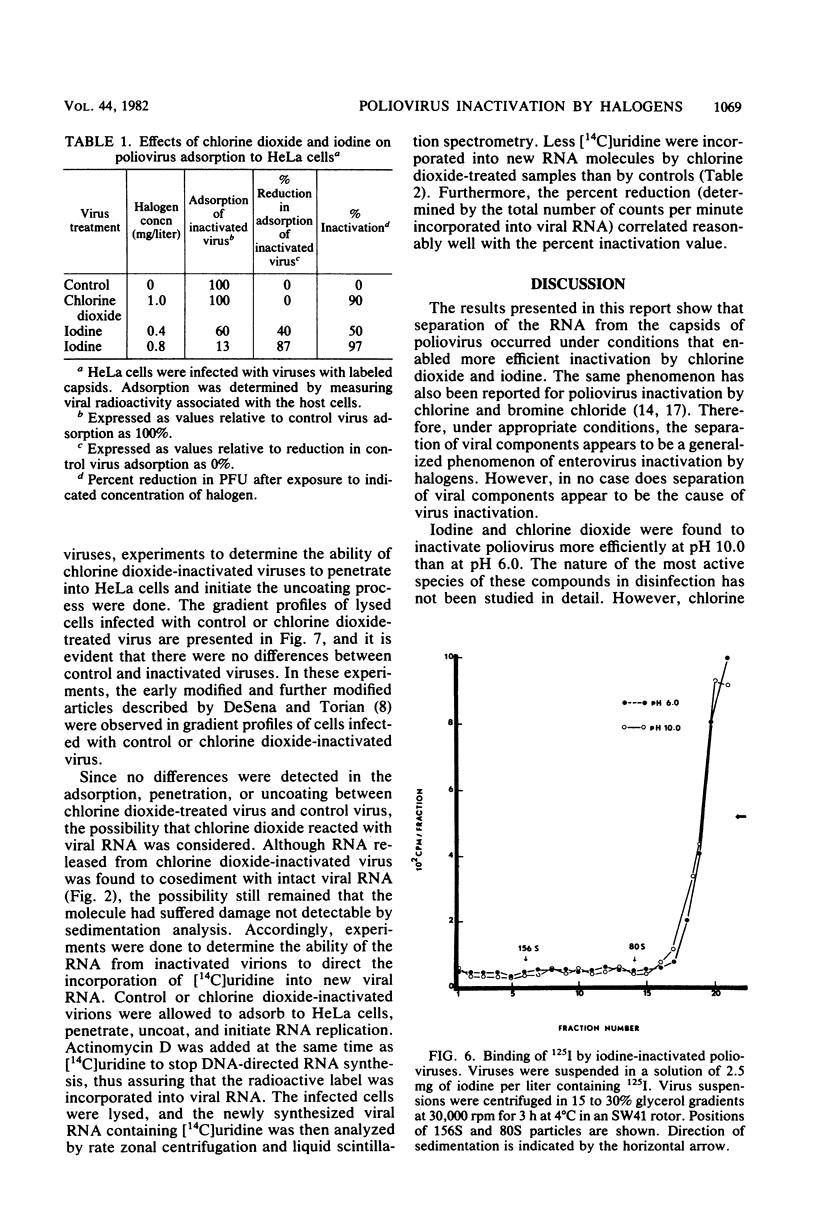
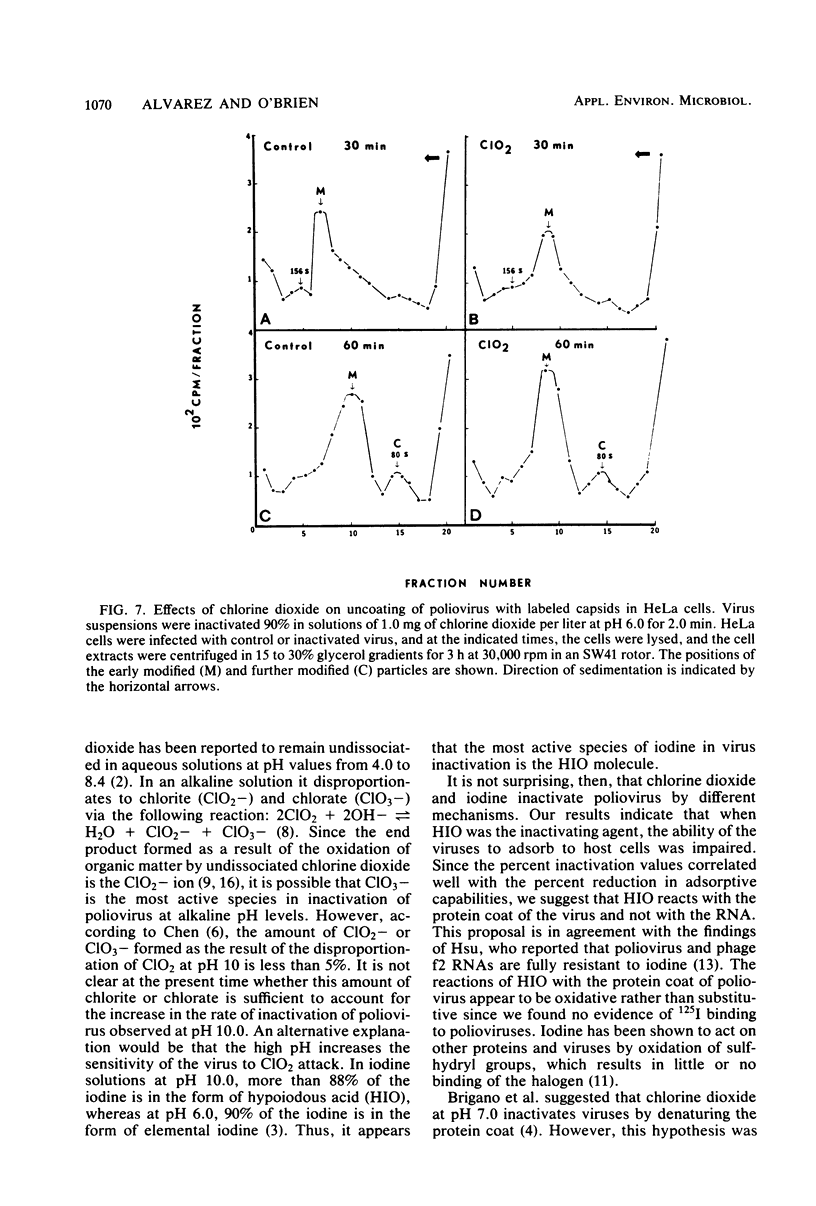
Chlorine dioxide and iodine inactivated poliovirus more efficiently at pH 10.0 than at pH 6.0. Sedimentation analyses of viruses inactivated by chlorine dioxide and iodine at pH 10.9 showed that viral RNA separated from the capsids, resulting in the conversion of virions from 156S structures to 80S particles. The RNAs release from both chlorine dioxide- and iodine-inactivated viruses cosedimented with intact 35S viral RNA. Both chlorine dioxide and iodine reacted with the capsid proteins of poliovirus and changed the pI from pH 7.0 to pH 5.8. However, the mechanisms of inactivation of poliovirus by chlorine dioxide and iodine were found to differ. Iodine inactivated viruses by impairing their ability to adsorb to HeLa cells, whereas chlorine dioxide-inactivated viruses showed a reduced incorporation of [14C]uridine into new viral RNA. We concluded, then, that chlorine dioxide inactivated poliovirus by reacting with the viral RNA and impairing the ability of the viral genome to act as a template for RNA synthesis.
Full text
PDF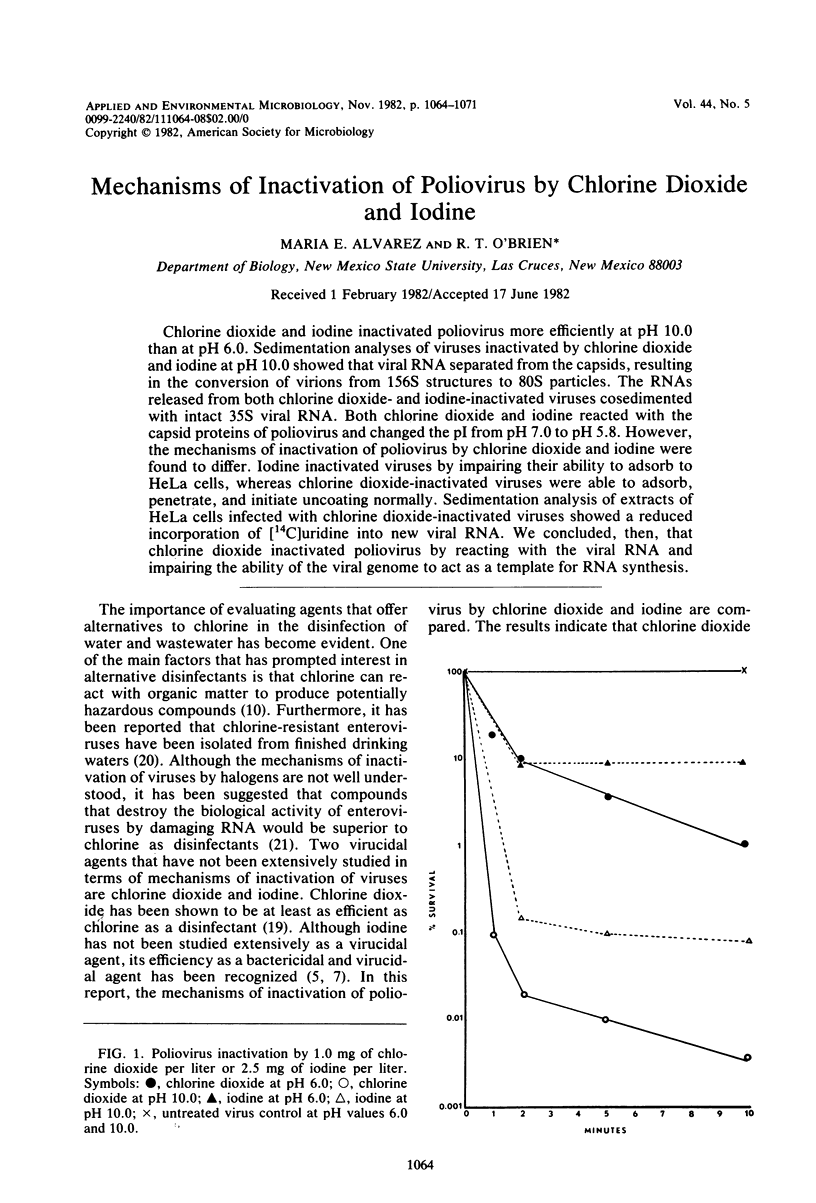

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez M. E., O'Brien R. T. Effects of chlorine concentration on the structure of poliovirus. Appl Environ Microbiol. 1982 Jan;43(1):237–239. doi: 10.1128/aem.43.1.237-239.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarde M. A., Israel B. M., Olivieri V. P., Granstrom M. L. Efficiency of chlorine dioxide as a bactericide. Appl Microbiol. 1965 Sep;13(5):776–780. doi: 10.1128/am.13.5.776-780.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer W. N., Kawata K., Krusé C. W. Chlorination and iodination of poliovirus and f2. J Water Pollut Control Fed. 1976 Jan;48(1):61–76. [PubMed] [Google Scholar]
- De Sena J., Torian B. Studies on the in vitro uncoating of poliovirus. III. Roles of membrane-modifying and -stabilizing factors in the generation of subviral particles. Virology. 1980 Jul 15;104(1):149–163. doi: 10.1016/0042-6822(80)90373-6. [DOI] [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H. The reaction of tobacco mosaic virus with iodine. J Biol Chem. 1955 Nov;217(1):373–381. [PubMed] [Google Scholar]
- Keswick B. H., Fujioka R. S., Loh P. C. Mechanism of poliovirus inactivation by bromine chloride. Appl Environ Microbiol. 1981 Nov;42(5):824–829. doi: 10.1128/aem.42.5.824-829.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel B. Characterization of type 1 poliovirus by electrophoretic analysis. Virology. 1971 Jun;44(3):554–568. doi: 10.1016/0042-6822(71)90369-2. [DOI] [PubMed] [Google Scholar]
- O'Brien R. T., Newman J. Structural and compositional changes associated with chlorine inactivation of polioviruses. Appl Environ Microbiol. 1979 Dec;38(6):1034–1039. doi: 10.1128/aem.38.6.1034-1039.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer P. T., Metcalf T. G., Sproul O. J. Chlorine resistance of poliovirus isolants recovered from drinking water. Appl Environ Microbiol. 1980 Dec;40(6):1115–1121. doi: 10.1128/aem.40.6.1115-1121.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager J. G., O'Brien R. T. Structural changes associated with poliovirus inactivation in soil. Appl Environ Microbiol. 1979 Oct;38(4):702–709. doi: 10.1128/aem.38.4.702-709.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


