Abstract
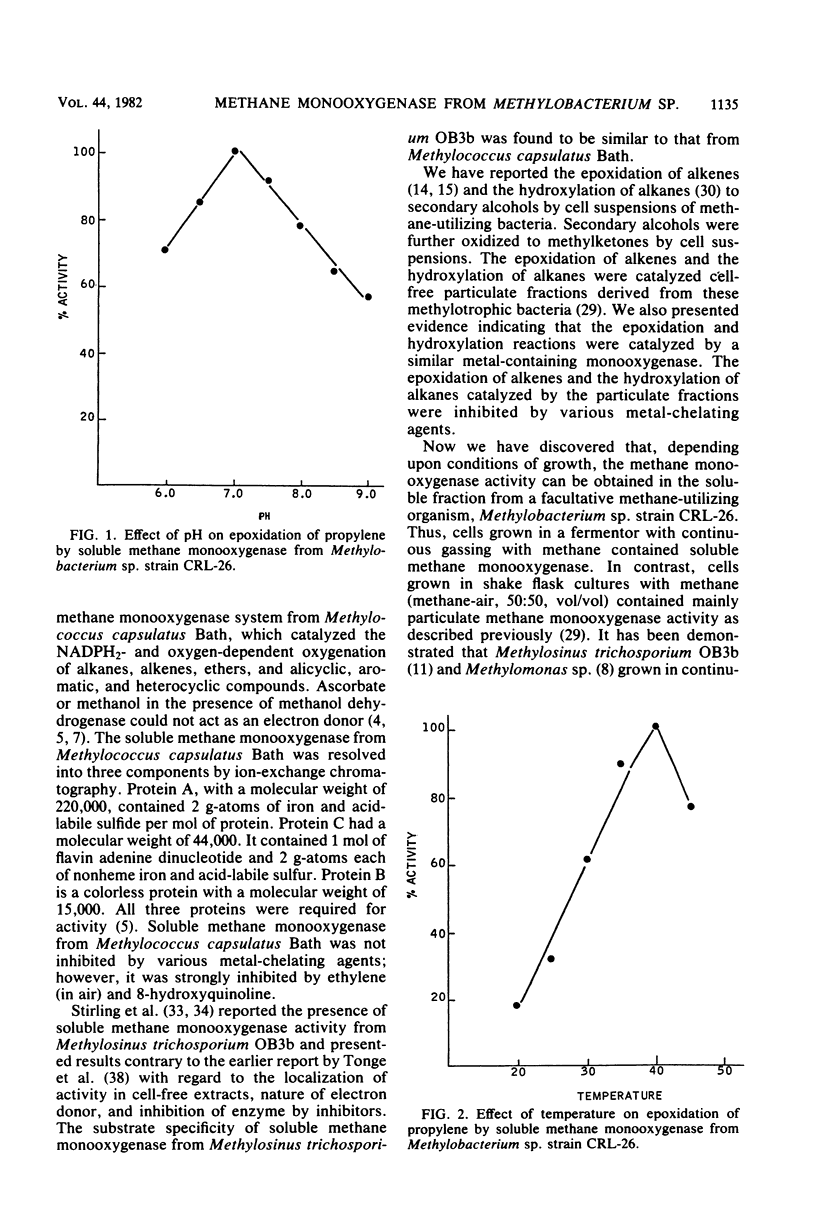
Methylobacterium sp. strain CRL-26 grown in a fermentor contained methane monooxygenase activity in soluble fractions. Soluble methane monooxygenase catalyzed the epoxidation/hydroxylation of a variety of hydrocarbons, including terminal alkenes, internal alkenes, substituted alkenes, branched-chain alkenes, alkanes (C1 to C8), substituted alkanes, branched-chain alkanes, carbon monoxide, ethers, and cyclic and aromatic compounds. The optimum pH and temperature for the epoxidation of propylene by soluble methane monooxygenase were found to be 7.0 and 40°C, respectively. Among various compounds tested, only NADH2 or NADPH2 could act as an electron donor. Formate and NAD+ (in the presence of formate dehydrogenase contained in the soluble fraction) or 2-butanol in the presence of NAD+ and secondary alcohol dehydrogenase generated the NADH2 required for the methane monooxygenase. Epoxidation of propylene catalyzed by methane monooxygenase was not inhibited by a range of potential inhibitors, including metal-chelating compounds and potassium cyanide. Sulfhydryl agents and acriflavin inhibited monooxygenase activity. Soluble methane monooxygenase was resolved into three components by ion-exchange chromatography. All three compounds are required for the epoxidation and hydroxylation reactions.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott B. J., Hou C. T. Oxidation of 1-alkenes to 1,2-epoxyalkanes by Pseudomonas oleovorans. Appl Microbiol. 1973 Jul;26(1):86–91. doi: 10.1128/am.26.1.86-91.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardini G., Jurtshuk P. The enzymatic hydroxylation of n-octane by Corynebacterium sp. strain 7E1C. J Biol Chem. 1970 Jun 10;245(11):2789–2796. [PubMed] [Google Scholar]
- Colby J., Dalton H. Characterization of the second prosthetic group of the flavoenzyme NADH-acceptor reductase (component C) of the methane mono-oxygenase from Methylococcus capsulatus (Bath). Biochem J. 1979 Mar 1;177(3):903–908. doi: 10.1042/bj1770903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J., Dalton H. Resolution of the methane mono-oxygenase of Methylococcus capsulatus (Bath) into three components. Purification and properties of component C, a flavoprotein. Biochem J. 1978 May 1;171(2):461–468. doi: 10.1042/bj1710461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J., Dalton H. Some properties of a soluble methane mono-oxygenase from Methylococcus capsulatus strain Bath. Biochem J. 1976 Aug 1;157(2):495–497. doi: 10.1042/bj1570495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J., Stirling D. I., Dalton H. The soluble methane mono-oxygenase of Methylococcus capsulatus (Bath). Its ability to oxygenate n-alkanes, n-alkenes, ethers, and alicyclic, aromatic and heterocyclic compounds. Biochem J. 1977 Aug 1;165(2):395–402. doi: 10.1042/bj1650395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boer W. E., Hazeu W. Observations on the fine structure of a methane-oxidizing bacterium. Antonie Van Leeuwenhoek. 1972;38(1):33–47. doi: 10.1007/BF02328075. [DOI] [PubMed] [Google Scholar]
- Ferenci T., Strom T., Quayle J. R. Oxidation of carbon monoxide and methane by Pseudomonas methanica. J Gen Microbiol. 1975 Nov;91(1):79–91. doi: 10.1099/00221287-91-1-79. [DOI] [PubMed] [Google Scholar]
- Foster J. W., Davis R. H. A methane-dependent coccus, with notes on classification and nomenclature of obligate, methane-utilizing bacteria. J Bacteriol. 1966 May;91(5):1924–1931. doi: 10.1128/jb.91.5.1924-1931.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins I. J., Hammond R. C., Sariaslani F. S., Best D., Davies M. M., Tryhorn S. E., Taylor F. Biotransformation of hydrocarbons and related compounds by whole organism suspensions of methane-grown methylosinus trichosporium OB 3b. Biochem Biophys Res Commun. 1979 Jul 27;89(2):671–677. doi: 10.1016/0006-291x(79)90682-x. [DOI] [PubMed] [Google Scholar]
- Higgins I. J., Quayle J. R. Oxygenation of methane by methane-grown Pseudomonas methanica and Methanomonas methanooxidans. Biochem J. 1970 Jun;118(2):201–208. doi: 10.1042/bj1180201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C. T., Patel R. N., Laski A. I., Marczak I., Barnabe N. Microbial oxidation of gaseous hydrocarbons: production of alcohols and methyl ketones from their corresponding n-alkanes by methylotrophic bacteria. Can J Microbiol. 1981 Jan;27(1):107–115. doi: 10.1139/m81-017. [DOI] [PubMed] [Google Scholar]
- Hou C. T., Patel R., Laskin A. I., Barnabe N. Microbial oxidation of gaseous hydrocarbons: epoxidation of C2 to C4 n-alkenes by methylotrophic bacteria. Appl Environ Microbiol. 1979 Jul;38(1):127–134. doi: 10.1128/aem.38.1.127-134.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEADBETTER E. R., FOSTER J. W. Bacterial oxidation of gaseous alkanes. Arch Mikrobiol. 1960;35:92–104. doi: 10.1007/BF00425597. [DOI] [PubMed] [Google Scholar]
- LEADBETTER E. R., FOSTER J. W. Studies on some methane-utilizing bacteria. Arch Mikrobiol. 1958;30(1):91–118. doi: 10.1007/BF00509229. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- May S. W., Abbott B. J. Enzymatic epoxidation. I. Alkene epoxidation by the -hydroxylation system of Pseudomonas oleovorans. Biochem Biophys Res Commun. 1972 Sep 5;48(5):1230–1234. doi: 10.1016/0006-291x(72)90842-x. [DOI] [PubMed] [Google Scholar]
- McKenna E. J., Coon M. J. Enzymatic omega-oxidation. IV. Purification and properties of the omega-hydroxylase of Pseudomonas oleovorans. J Biol Chem. 1970 Aug 10;245(15):3882–3889. [PubMed] [Google Scholar]
- NASH T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem J. 1953 Oct;55(3):416–421. doi: 10.1042/bj0550416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. N., Hou C. T., Felix A. Microbial oxidation of methane and methanol: crystallization of methanol dehydrogenase and properties of holo- and apomethanol dehydrogenase from Methylomonas methanica. J Bacteriol. 1978 Feb;133(2):641–649. doi: 10.1128/jb.133.2.641-649.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. N., Hou C. T., Felix A. Microbial oxidation of methane and methanol: isolation of methane-utilizing bacteria and characterization of a facultative methane-utilizing isolate. J Bacteriol. 1978 Oct;136(1):352–358. doi: 10.1128/jb.136.1.352-358.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. N., Hou C. T., Laskin A. I., Derelanko P., Felix A. Microbial production of methyl ketones. Purification and properties of a secondary alcohol dehydrogenase from yeast. Eur J Biochem. 1979 Nov;101(2):401–406. doi: 10.1111/j.1432-1033.1979.tb19732.x. [DOI] [PubMed] [Google Scholar]
- Patel R. N., Hou C. T., Laskin A. I., Felix A., Derelanko P. Microbial Oxidation of Gaseous Hydrocarbons: Production of Secondary Alcohols from Corresponding n-Alkanes by Methane-Utilizing Bacteria. Appl Environ Microbiol. 1980 Apr;39(4):720–726. doi: 10.1128/aem.39.4.720-726.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. N., Hou C. T., Laskin A. I., Felix A., Derelanko P. Microbial oxidation of gaseous hydrocarbons. II. Hydroxylation of alkanes and epoxidation of alkenes by cell-free particulate fractions of methane-utilizing bacteria. J Bacteriol. 1979 Aug;139(2):675–679. doi: 10.1128/jb.139.2.675-679.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R., Hou C. T., Felix A. Inhibition of dimethyl ether and methane oxidation in Methylococcus capsulatus and Methylosinus trichosporium. J Bacteriol. 1976 May;126(2):1017–1019. doi: 10.1128/jb.126.2.1017-1019.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribbons D. W. Oxidation of C1 Compounds by Particulate fractions from Methylococcus capsulatus: distribution and properties of methane-dependent reduced nicotinamide adenine dinucleotide oxidase (methane hydroxylase). J Bacteriol. 1975 Jun;122(3):1351–1363. doi: 10.1128/jb.122.3.1351-1363.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling D. I., Colby J., Dalton H. A comparison of the substrate and electron-donor specificities of the methane mono-oxygenases from three strains of methane-oxidizing bacteria. Biochem J. 1979 Jan 1;177(1):361–364. doi: 10.1042/bj1770361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling D. I., Dalton H. Properties of the methane mono-oxygenase from extracts of Methylosinus trichosporium OB3b and evidence for its similarity to the enzyme from Methylococcus capsulatus (Bath). Eur J Biochem. 1979 May 2;96(1):205–212. doi: 10.1111/j.1432-1033.1979.tb13030.x. [DOI] [PubMed] [Google Scholar]
- Thomson A. W., O'Neill J. G., Wilkinson J. F. Acetone production by methylobacteria. Arch Microbiol. 1976 Sep 1;109(3):243–246. doi: 10.1007/BF00446635. [DOI] [PubMed] [Google Scholar]
- Tonge G. M., Harrison D. E., Higgins I. J. Purification and properties of the methane mono-oxygenase enzyme system from Methylosinus trichosporium OB3b. Biochem J. 1977 Feb 1;161(2):333–344. doi: 10.1042/bj1610333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonge G. M., Harrison D. E., Knowles C. J., Higgins I. J. Properties and partial purification of the methane-oxidising enzyme system from Methylosinus trichosporium. FEBS Lett. 1975 Oct 15;58(1):293–299. doi: 10.1016/0014-5793(75)80282-1. [DOI] [PubMed] [Google Scholar]
- VAN DER LINDEN A. C. EPOXIDATION OF ALPHA-OLEFINS BY HEPTANE-GROWN PSEUDOMONAS CELLS. Biochim Biophys Acta. 1963 Sep 3;77:157–159. doi: 10.1016/0006-3002(63)90484-0. [DOI] [PubMed] [Google Scholar]



