Abstract
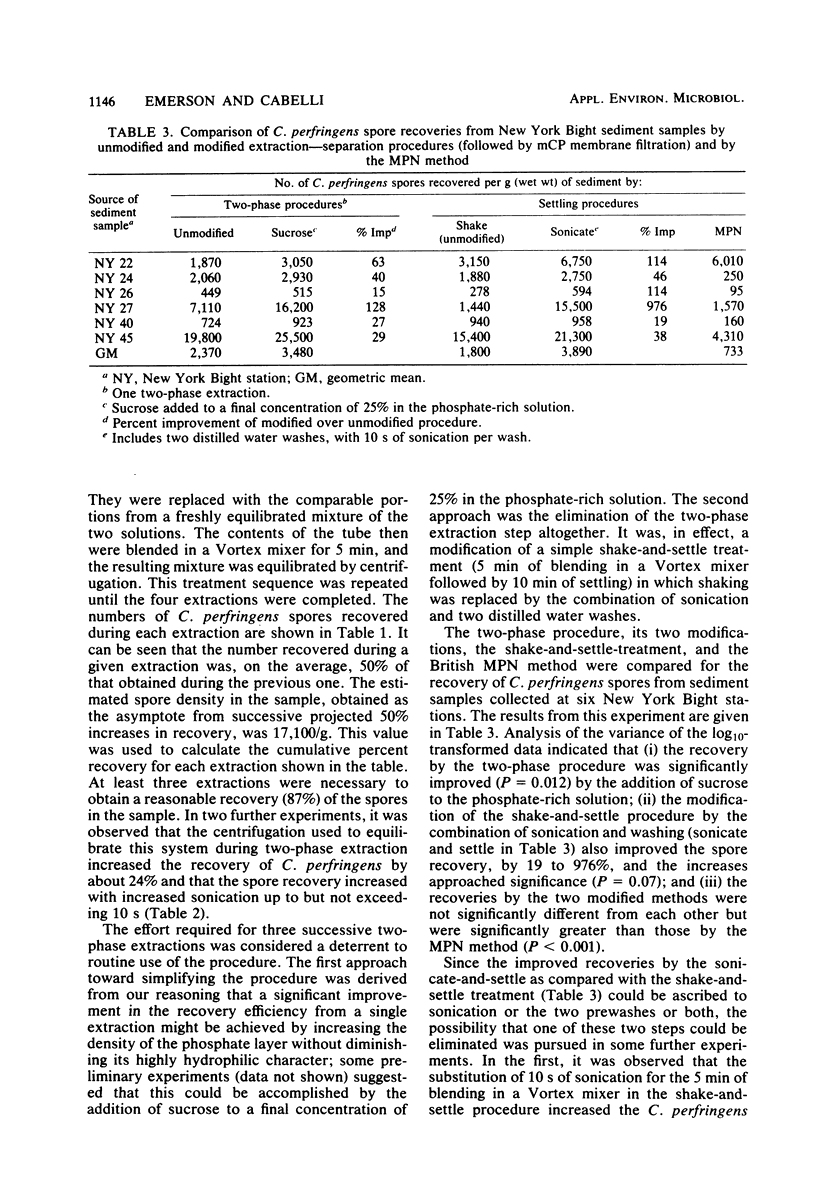
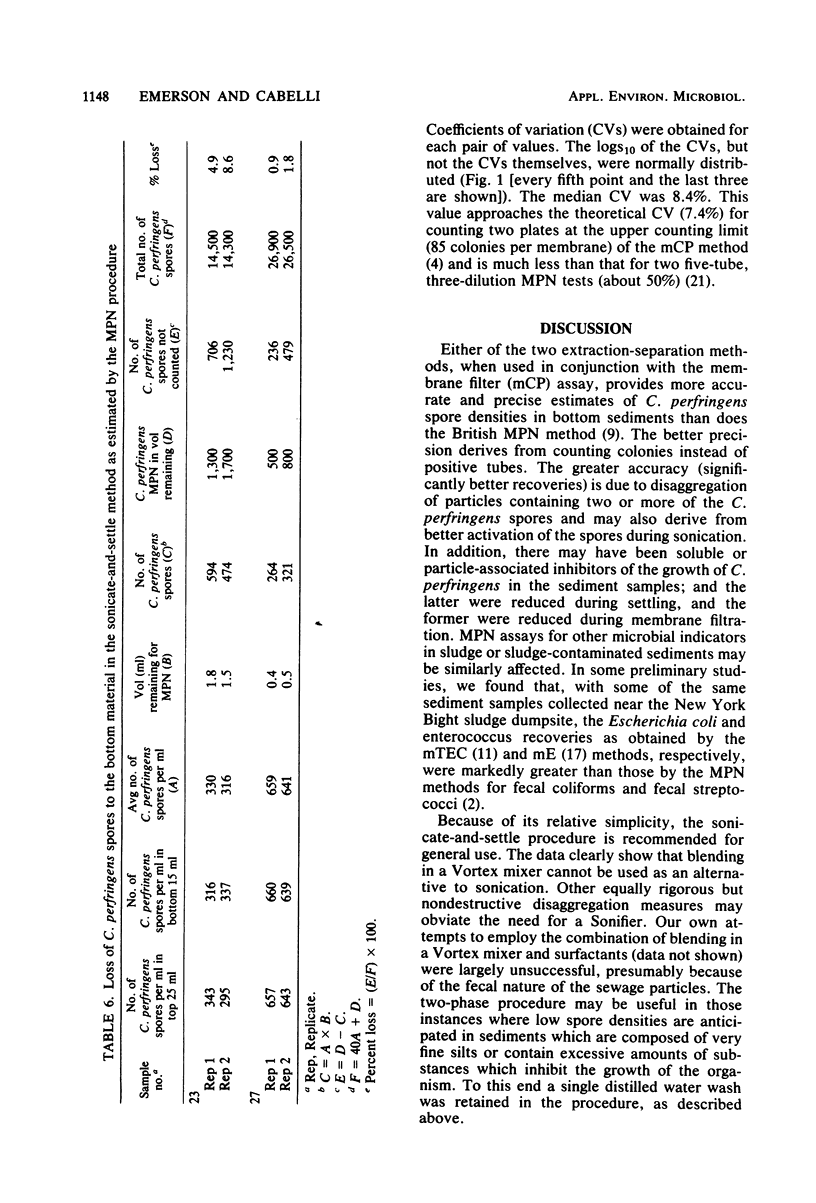
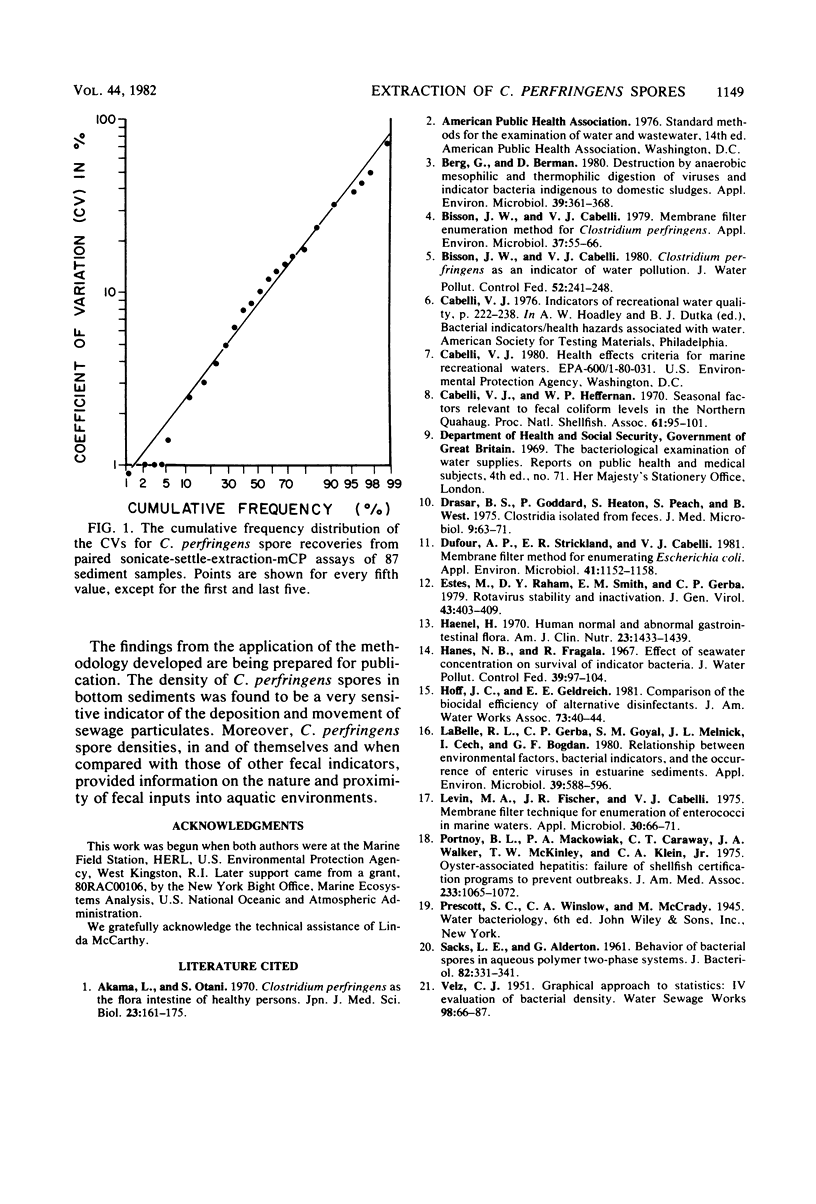
Two extraction-separation procedures were developed and evaluated for use in conjunction with the mCP membrane filter method for the enumeration of Clostridium perfringens spores in bottom sediments. In the more facile of the two procedures, a distilled-water suspension of the sediment sample is pulse sonicated for 10 s and allowed to settle. Portions of the supernatant are then removed for membrane filtration. This procedure is recommended for general use. The more complicated procedure is recommended for situations in which the presence of high levels of toxic materials is suspected or in which relatively low spore densities are present in fine silts. In this procedure, sonication is followed by a distilled water wash. The centrifuged sediment is resuspended in distilled water and mixed with the components of a two-phase separation system (50% polyethylene glycol in distilled water and 25% sucrose in 3 M phosphate buffer [pH 7.1]). After equilibration of the system and low-speed centrifugation, the top phase and interphase are removed, mixed, and membrane filtered. The recoveries of C. perfringens spores by the two procedures, when used in conjunction with the mCP method, were comparable to each other and significantly greater than those by the British most-probable-number method. It was estimated that more than 85% of the spores were recovered by the procedures. The precision of the sonicate-and-settle-mCP procedure was markedly better than that obtained theoretically by the most-probable-number method and approached that theoretically attributable to counting an average of 85 colonies on each of two plates.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akama K., Otani S. Clostridium perfringens as the flora in the intestine of healthy persons. Jpn J Med Sci Biol. 1970 Jun;23(3):161–175. doi: 10.7883/yoken1952.23.161. [DOI] [PubMed] [Google Scholar]
- Berg G., Berman D. Destruction by anaerobic mesophilic and thermophilic digestion of viruses and indicator bacteria indigenous to domestic sludges. Appl Environ Microbiol. 1980 Feb;39(2):361–368. doi: 10.1128/aem.39.2.361-368.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson J. W., Cabelli V. J. Clostridium perfringens as a water pollution indicator. J Water Pollut Control Fed. 1980 Feb;52(2):241–248. [PubMed] [Google Scholar]
- Bisson J. W., Cabelli V. J. Membrane filter enumeration method for Clostridium perfringens. Appl Environ Microbiol. 1979 Jan;37(1):55–66. doi: 10.1128/aem.37.1.55-66.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drasar B. S., Goddard P., Heaton S., Peach S., West B. Clostridia isolated from faeces. J Med Microbiol. 1976 Feb;9(1):63–71. doi: 10.1099/00222615-9-1-63. [DOI] [PubMed] [Google Scholar]
- Dufour A. P., Strickland E. R., Cabelli V. J. Membrane filter method for enumerating Escherichia coli. Appl Environ Microbiol. 1981 May;41(5):1152–1158. doi: 10.1128/aem.41.5.1152-1158.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Graham D. Y., Smith E. M., Gerba C. P. Rotavirus stability and inactivation. J Gen Virol. 1979 May;43(2):403–409. doi: 10.1099/0022-1317-43-2-403. [DOI] [PubMed] [Google Scholar]
- Haenel H. Human normal and abnormal gastrointestinal flora. Am J Clin Nutr. 1970 Nov;23(11):1433–1439. doi: 10.1093/ajcn/23.11.1433. [DOI] [PubMed] [Google Scholar]
- Hanes N. B., Fragala R. Effect of seawater concentration on survival of indicator bacteria. J Water Pollut Control Fed. 1967 Jan;39(1):97–104. [PubMed] [Google Scholar]
- LaBelle R. L., Gerba C. P., Goyal S. M., Melnick J. L., Cech I., Bogdan G. F. Relationships between environmental factors, bacterial indicators, and the occurrence of enteric viruses in estuarine sediments. Appl Environ Microbiol. 1980 Mar;39(3):588–596. doi: 10.1128/aem.39.3.588-596.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M. A., Fischer J. R., Cabelli V. J. Membrane filter technique for enumeration of enterococci in marine waters. Appl Microbiol. 1975 Jul;30(1):66–71. doi: 10.1128/am.30.1.66-71.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy B. L., Mackowiak P. A., Caraway C. T., Walker J. A., McKinley T. W., Klein C. A., Jr Oyster-associated hepatitis. Failure of shellfish certification programs to prevent outbreaks. JAMA. 1975 Sep 8;233(10):1065–1068. doi: 10.1001/jama.233.10.1065. [DOI] [PubMed] [Google Scholar]


