Abstract
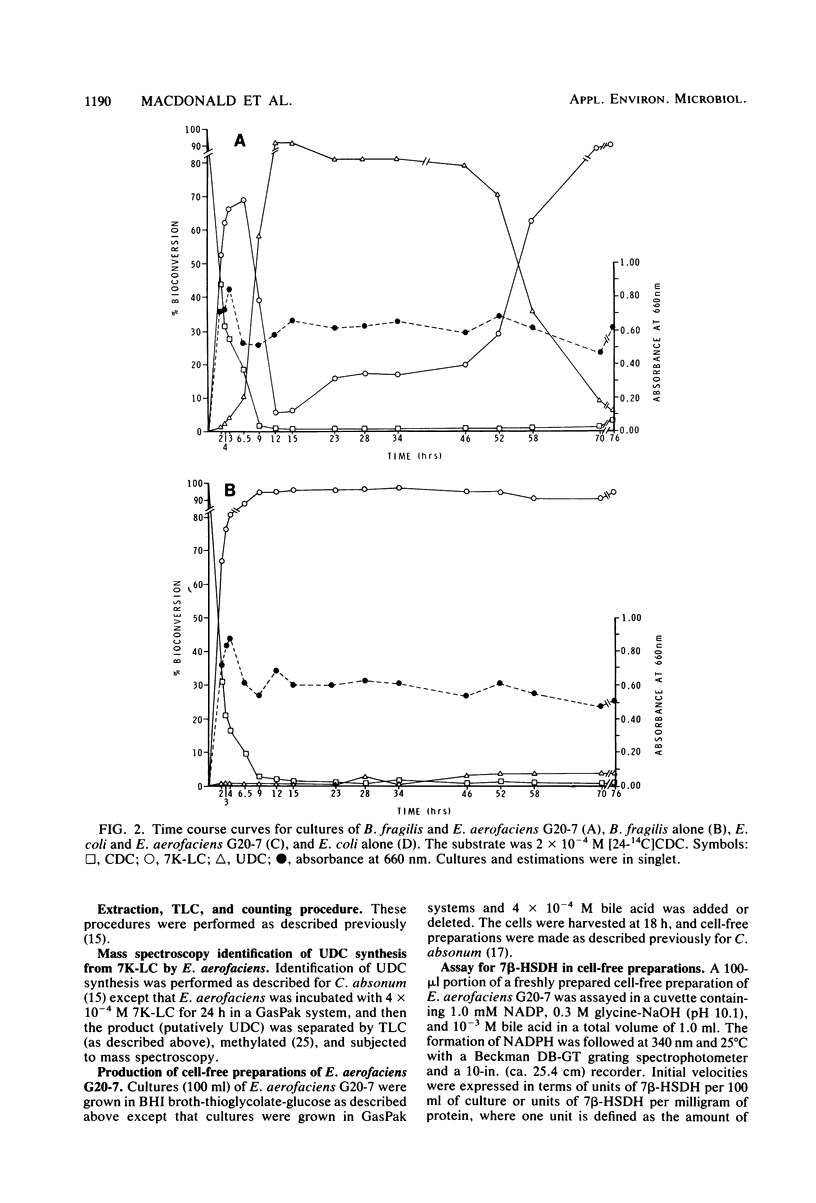
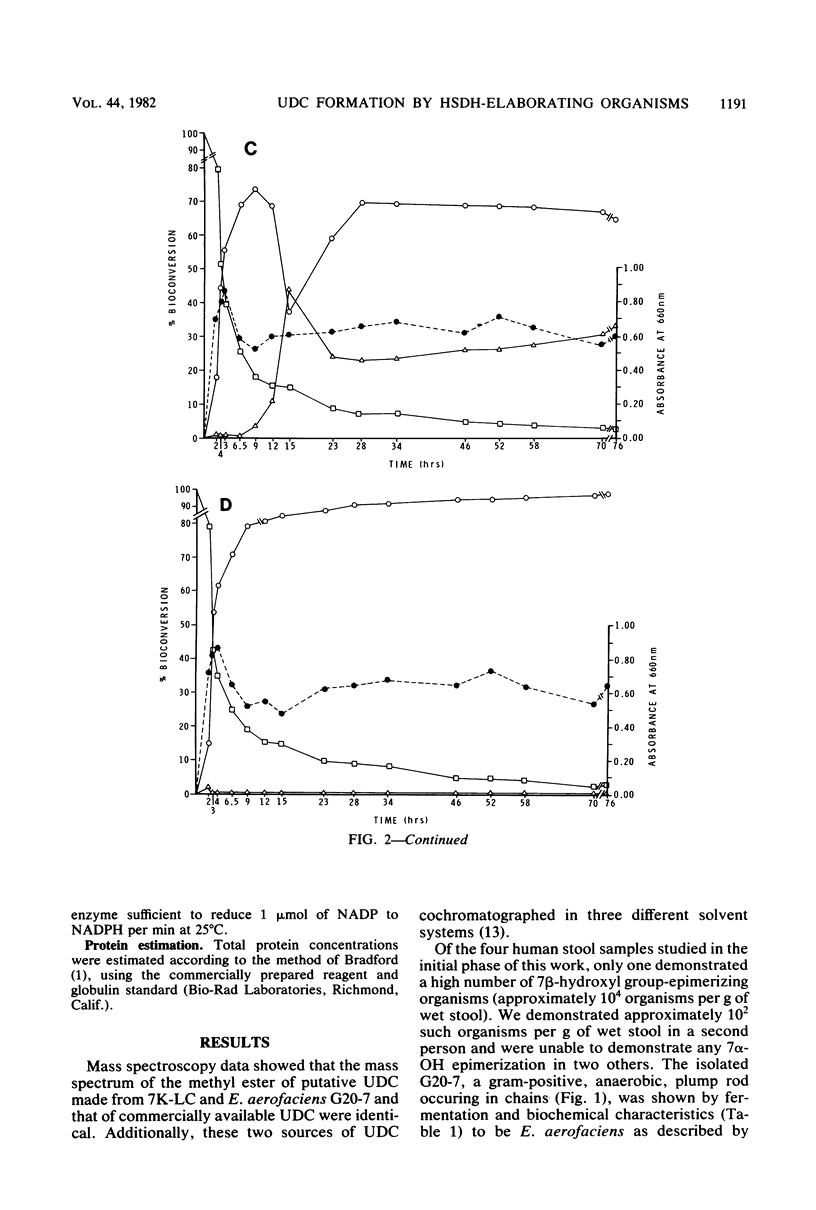
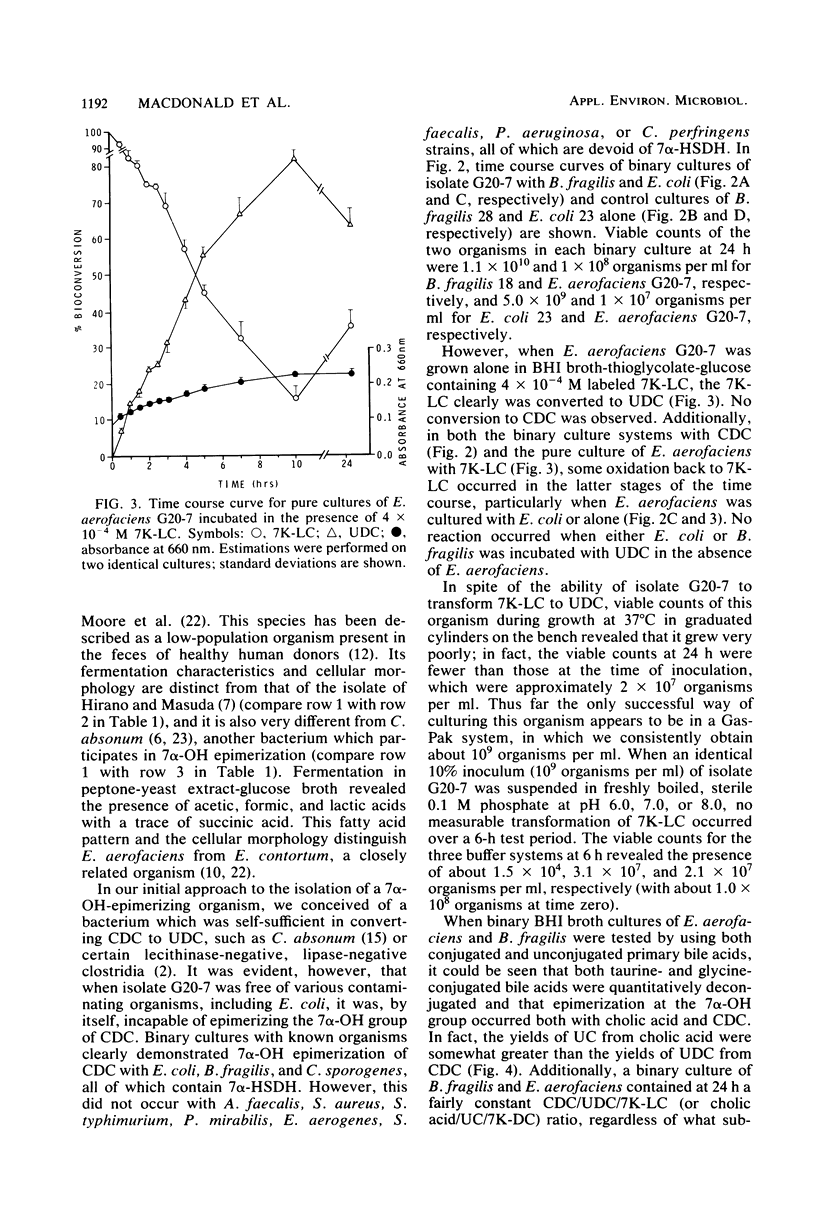
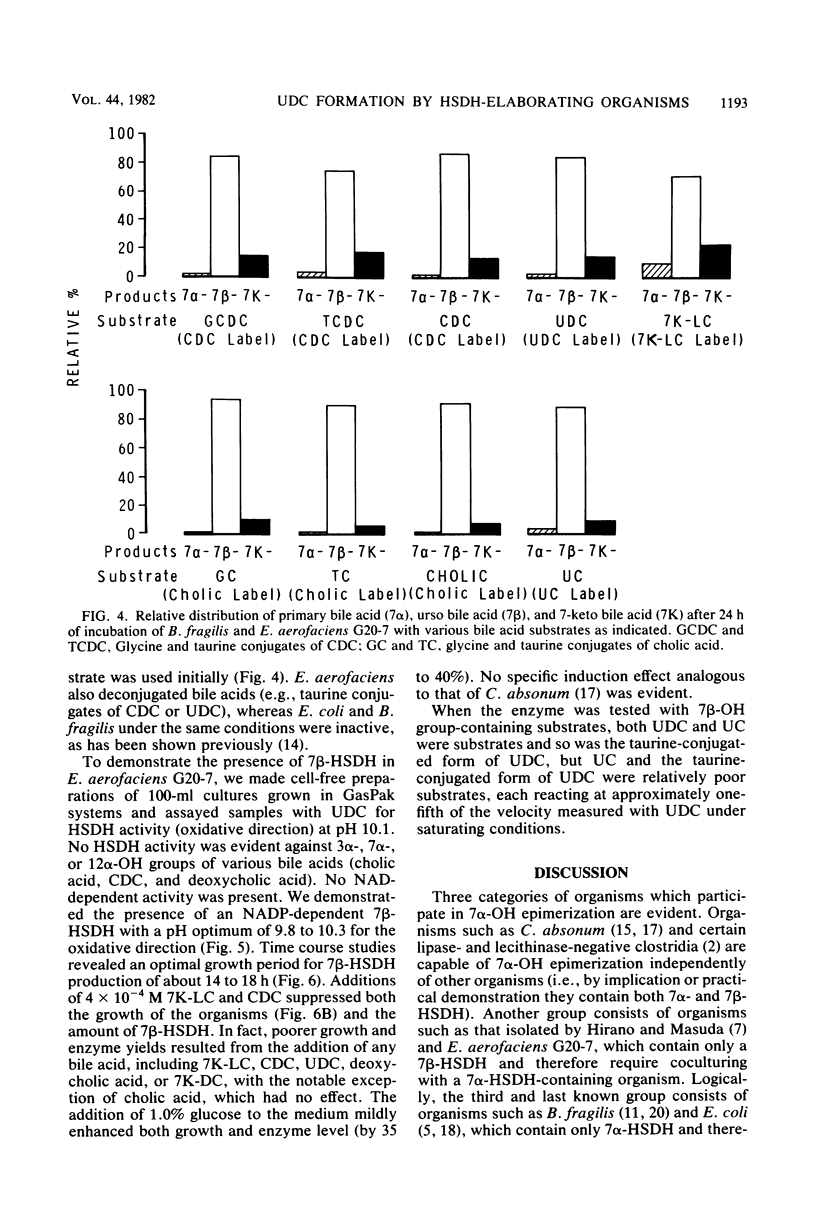
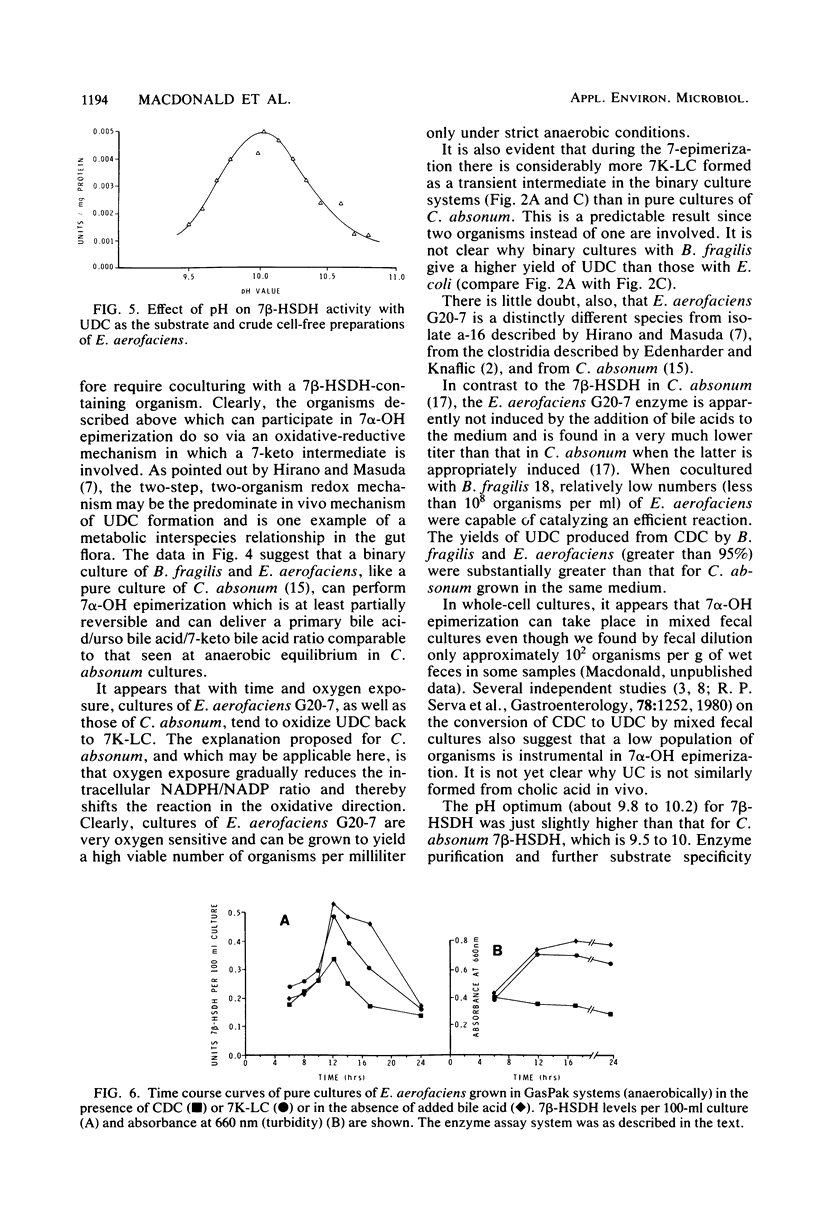
A gram-positive, anaerobic, chain-forming, rod-shaped anaerobe (isolate G20-7) was isolated from normal human feces. This organism was identified by cellular morphology as well as fermentative and biochemical data as Eubacterium aerofaciens. When isolate G20-7 was grown in the presence of Bacteroides fragilis or Escherichia coli (or another 7 alpha-hydroxysteroid dehydrogenase producer) and chenodeoxycholic acid, ursodeoxycholic acid produced. Time course curves revealed that 3 alpha-hydroxy-7-keto-5 beta-cholanoic acid produced by B. fragilis or E. coli or introduced into the medium as a pure substance was reduced by G20-7 specifically to ursodeoxycholic acid. The addition of glycine- and taurine-conjugated primary bile acids (chenodeoxycholic and cholic acids) and other bile acids to binary cultures of B. fragilis and G20-7 revealed that (i) both conjugates were hydrolyzed to give free bile acids, (ii) ursocholic acid (3 alpha, 7 beta, 12 alpha-trihydroxy-5 beta-cholanoic acid) was produced when conjugated (or free) cholic acid was the substrate, and (iii) the epimerization reaction was at least partially reversible. Corroborating these observations, an NADP-dependent 7 beta-hydroxysteroid dehydrogenase (reacting specifically with 7 beta-OH-groups) was demonstrated in cell-free preparations of isolate G20-7; production of the enzyme was optimal at between 12 and 18 h of growth. This enzyme, when measured in the oxidative direction, was active with ursodeoxycholic acid, ursocholic acid, and the taurine conjugate of ursodeoxycholic acid (but not with chenodeoxycholic, deoxycholic, or cholic acids) and displayed an optimal pH range of 9.8 to 10.2
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Edenharder R., Knaflic T. Epimerization of chenodeoxycholic acid to ursodeoxycholic acid by human intestinal lecithinase-lipase-negative Clostridia. J Lipid Res. 1981 May;22(4):652–658. [PubMed] [Google Scholar]
- Fedorowski T., Salen G., Tint G. S., Mosbach E. Transformation of chenodeoxycholic acid and ursodeoxycholic acid by human intestinal bacteria. Gastroenterology. 1979 Nov;77(5):1068–1073. [PubMed] [Google Scholar]
- Ferrari A., Scolatico C., Beretta L. On the mechanism of cholic acid 7alpha-dehydroxylation by a Clostridium bifermentans cell-free extract. FEBS Lett. 1977 Mar 15;75(1):166–168. doi: 10.1016/0014-5793(77)80077-x. [DOI] [PubMed] [Google Scholar]
- Haslewood E. S., Haslewood G. A. The specificity of a 7 alpha-hydroxy steroid dehydrogenase from Escherichia coli. Biochem J. 1976 Jul 1;157(1):207–210. doi: 10.1042/bj1570207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayase M., Mitsui N., Tamai K., Nakamura S., Nishida S. Isolation of Clostridium absonum and its cultural and biochemical properties. Infect Immun. 1974 Jan;9(1):15–19. doi: 10.1128/iai.9.1.15-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S., Masuda N. Epimerization of the 7-hydroxy group of bile acids by the combination of two kinds of microorganisms with 7 alpha- and 7 beta-hydroxysteroid dehydrogenase activity, respectively. J Lipid Res. 1981 Sep;22(7):1060–1068. [PubMed] [Google Scholar]
- Hirano S., Masuda N., Oda H. In vitro transformation of chenodeoxycholic acid and ursodeoxycholic acid by human intestinal flora, with particular reference to the mutual conversion between the two bile acids. J Lipid Res. 1981 Jul;22(5):735–743. [PubMed] [Google Scholar]
- Hirano S., Nakama R., Tamaki M., Masuda N., Oda H. Isolation and characterization of thirteen intestinal microorganisms capable of 7 alpha-dehydroxylating bile acids. Appl Environ Microbiol. 1981 Mar;41(3):737–745. doi: 10.1128/aem.41.3.737-745.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylemon P. B., Sherrod J. A. Multiple forms of 7-alpha-hydroxysteroid dehydrogenase in selected strains of Bacteroides fragilis. J Bacteriol. 1975 May;122(2):418–424. doi: 10.1128/jb.122.2.418-424.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornhof H. J., Richardson N. J., Wall D. M., Moore W. E. Fecal bacteria in South African rural blacks and other population groups. Isr J Med Sci. 1979 Apr;15(4):335–340. [PubMed] [Google Scholar]
- MacDonald I. A., Bishop J. M., Mahony D. E., Williams C. N. Convenient non-chromatographic assays for the microbial deconjugation and 7alpha-OH bioconversion of taurocholate. Appl Microbiol. 1975 Oct;30(4):530–535. doi: 10.1128/am.30.4.530-535.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald I. A., Roach P. D. Bile induction of 7 alpha- and 7 beta-hydroxysteroid dehydrogenases in Clostridium absonum. Biochim Biophys Acta. 1981 Aug 24;665(2):262–269. doi: 10.1016/0005-2760(81)90011-4. [DOI] [PubMed] [Google Scholar]
- Macdonald I. A. Detection of bile salts with Komarowsky's reagent and group specific dehydrogenases. J Chromatogr. 1977 Jun 11;136(2):348–352. doi: 10.1016/s0021-9673(00)86292-5. [DOI] [PubMed] [Google Scholar]
- Macdonald I. A., Hutchison D. M., Forrest T. P. Formation of urso- and ursodeoxy-cholic acids from primary bile acids by Clostridium absonum. J Lipid Res. 1981 Mar;22(3):458–466. [PubMed] [Google Scholar]
- Macdonald I. A., Meier E. C., Mahony D. E., Costain G. A. 3alpha-, 7alpha- and 12alpha-hydroxysteroid dehydrogenase activities from Clostridium perfringens. Biochim Biophys Acta. 1976 Nov 19;450(2):142–153. doi: 10.1016/0005-2760(76)90086-2. [DOI] [PubMed] [Google Scholar]
- Macdonald I. A., Williams C. N., Mahony D. E. 7Alpha-hydroxysteroid dehydrogenase from Escherichia coli B: preliminary studies. Biochim Biophys Acta. 1973 Jun 6;309(2):243–253. doi: 10.1016/0005-2744(73)90022-3. [DOI] [PubMed] [Google Scholar]
- Macdonald I. A., Williams C. N., Mahony D. E. A 3 alpha- and 7 alpha-hydroxysteroid dehydrogenase assay for conjugated dihydroxy-bile acid mixtures. Anal Biochem. 1974 Jan;57(1):127–136. doi: 10.1016/0003-2697(74)90059-1. [DOI] [PubMed] [Google Scholar]
- Macdonald I. A., Williams C. N., Mahony D. E., Christie W. M. NAD- and NADP-dependent 7alpha-hydroxysteroid dehydrogenases from bacteroides fragilis. Biochim Biophys Acta. 1975 Mar 28;384(1):12–24. doi: 10.1016/0005-2744(75)90091-1. [DOI] [PubMed] [Google Scholar]
- Mahony D. E., Meier C. E., Macdonald I. A., Holdeman L. V. Bile salt degradation by nonfermentative clostridia. Appl Environ Microbiol. 1977 Oct;34(4):419–423. doi: 10.1128/aem.34.4.419-423.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwag E. J., Hylemon P. B. 7alpha-Dehydroxylation of cholic acid and chenodeoxycholic acid by Clostridium leptum. J Lipid Res. 1979 Mar;20(3):325–333. [PubMed] [Google Scholar]
- Van Berge Henegouwen G. P., Ruben A., Brandt K. H. Quantitative analysis of bile acids in serum and bile, using gas--liquid chromatography. Clin Chim Acta. 1974 Aug 20;54(3):249–261. doi: 10.1016/0009-8981(74)90243-5. [DOI] [PubMed] [Google Scholar]
- White B. A., Cacciapuoti A. F., Fricke R. J., Whitehead T. R., Mosbach E. H., Hylemon P. B. Cofactor requiremets for 7 alpha-dehydroxylation of cholic and chenodeoxycholic acid in cell extracts of the intestinal anaerobic bacterium, Eubacterium species V.P.I. 13708. J Lipid Res. 1981 Aug;22(6):891–898. [PubMed] [Google Scholar]
- White B. A., Lipsky R. L., Fricke R. J., Hylemon P. B. Bile acid induction specificity of 7 alpha-dehydroxylase activity in an intestinal Eubacterium species. Steroids. 1980 Jan;35(1):103–109. doi: 10.1016/0039-128x(80)90115-4. [DOI] [PubMed] [Google Scholar]