Abstract
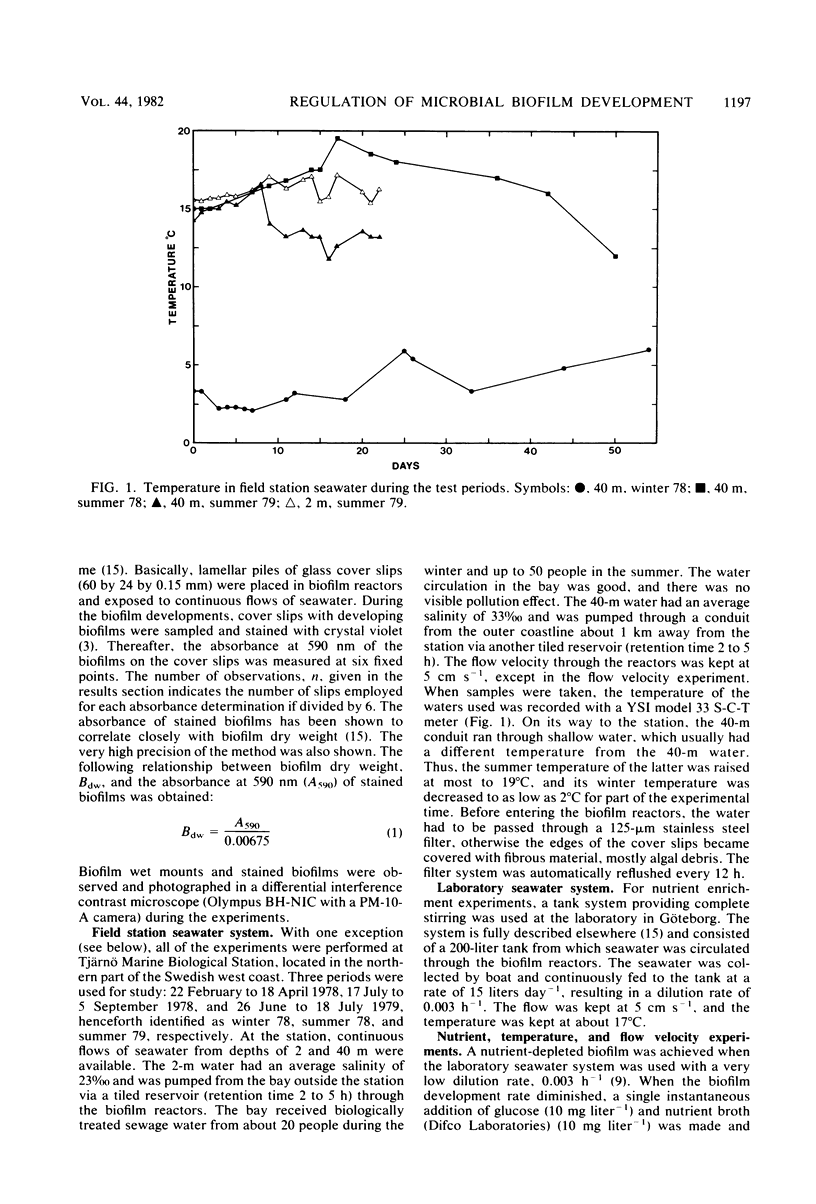
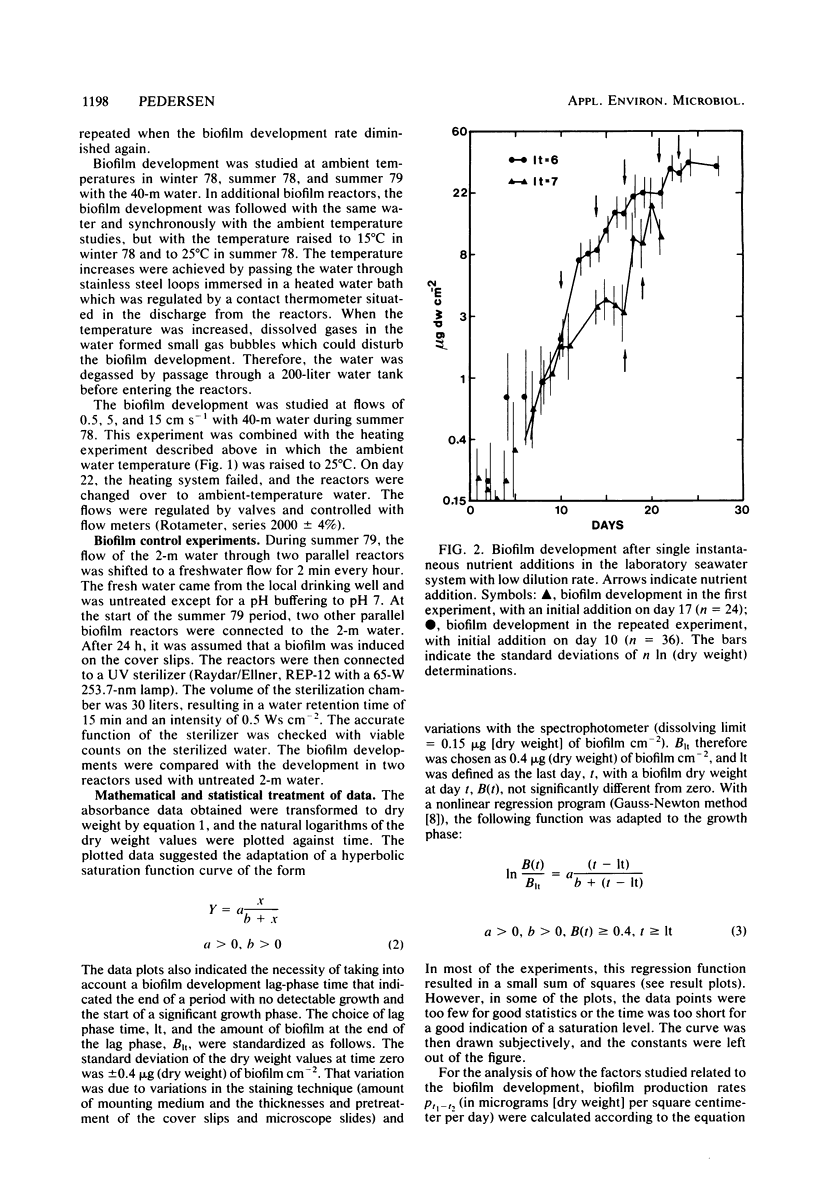
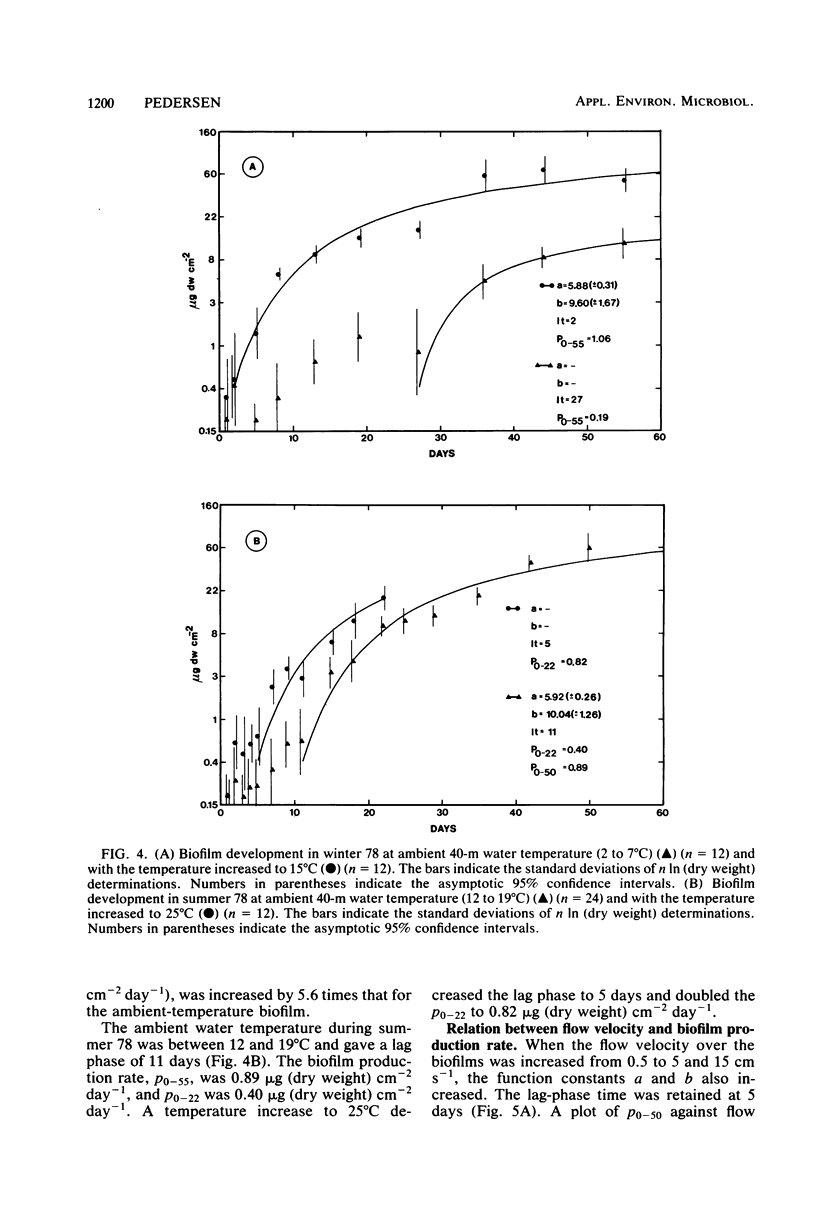
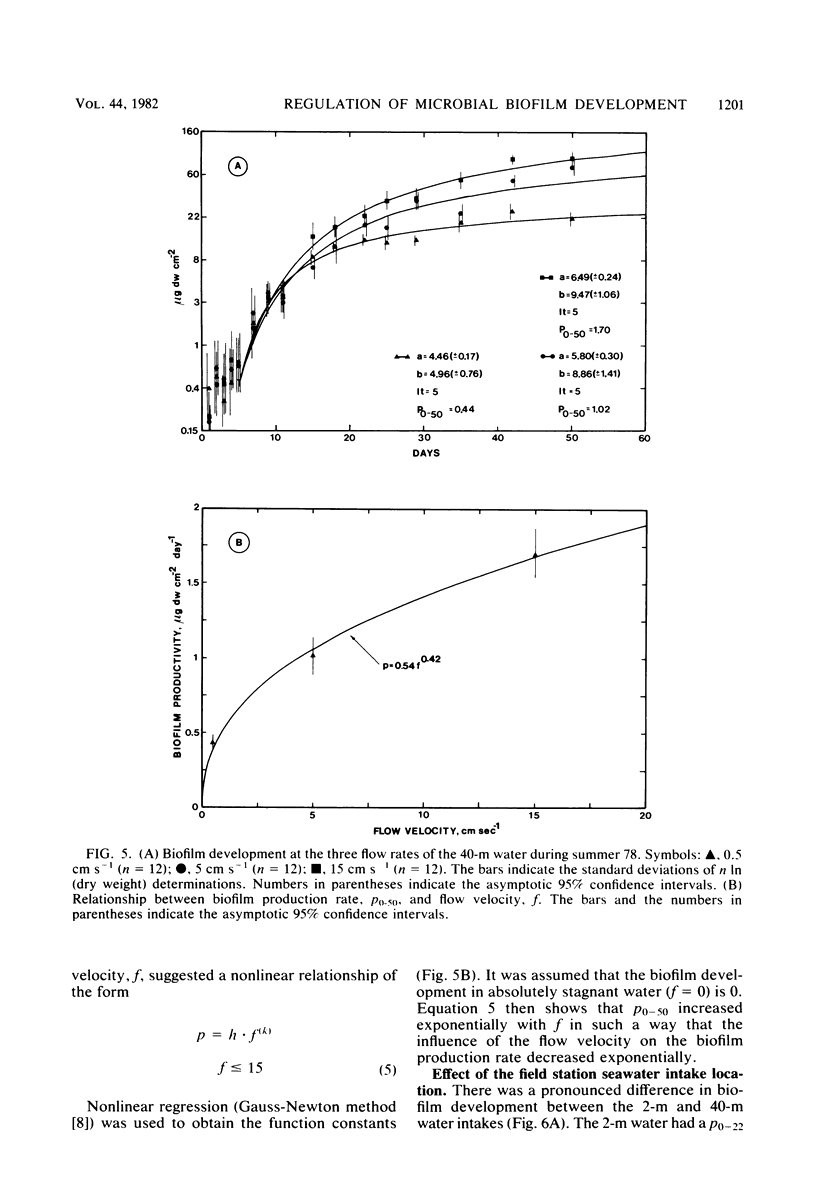
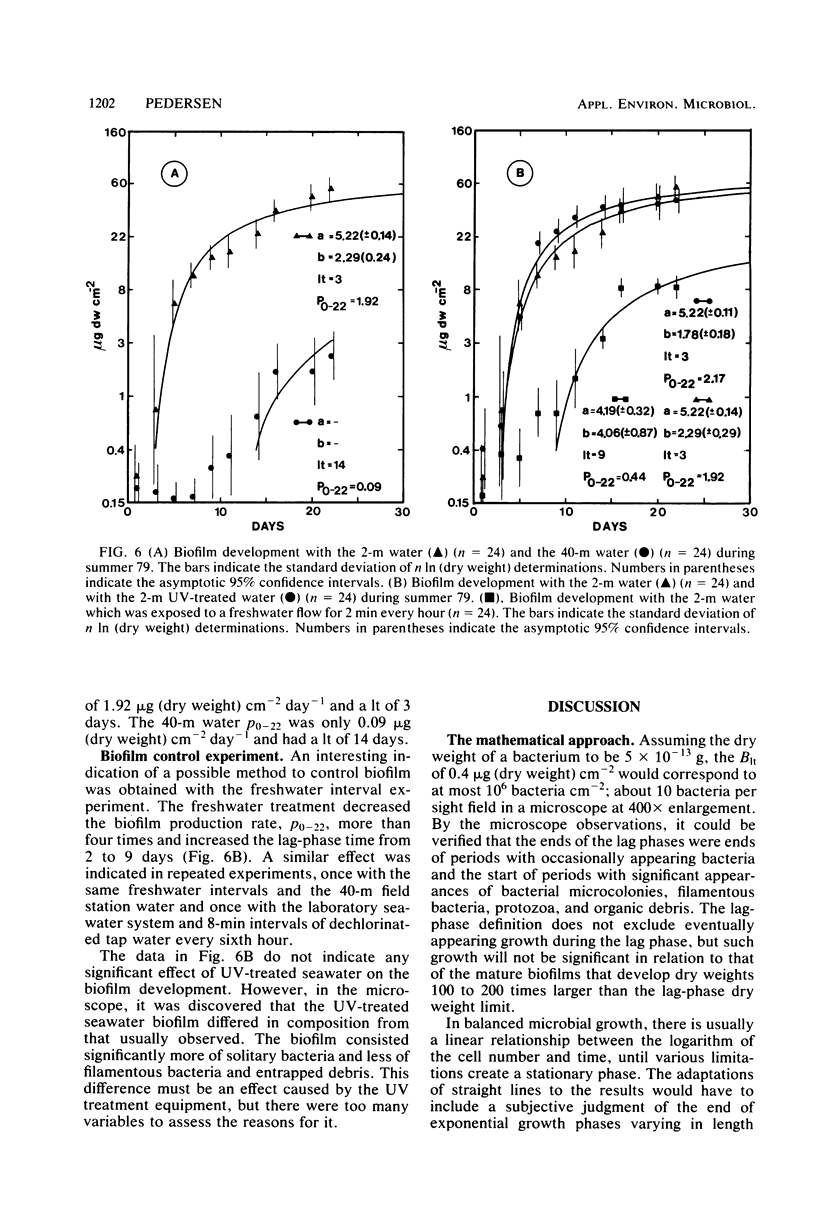
Microbial biofilm development was followed under growth conditions similar to those of a projected salinity power plant. Microscope glass cover slips were piled in biofilm reactors to imitate the membrane stacks in such a plant. A staining technique closely correlating absorbance values with biofilm dry weight was used for the study. Generally, the biofilms consisted of solitary and filamentous bacteria which were evenly distributed with considerable amounts of various protozoa and entrapped debris of organic origin. Protozoa predation was shown to decrease the amount of biofilm produced. The biofilm development lag phase was longer at lower temperatures. The subsequent growth phase was approximately arithmetic until stationary phase appeared. Adaptation of a hyperbolic saturation function gave curves that agreed well with the logarithm of the amount of biofilm as a function of time. Increased flow velocity, temperature, and nutrient concentration increased the biofilm production rate. An exponential relationship was shown between biofilm production rate and flow velocity within the range of 0 to 15 cm s−1. Intervals in which the biofilms were exposed to fresh water decreased the biofilm production rate more than four times. If the cover slips were inoculated with untreated seawater for 24 h, subsequent UV treatment had an insignificant effect on the biofilm formation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aftring R. P., Taylor B. F. Assessment of microbial fouling in an ocean thermal energy conversion experiment. Appl Environ Microbiol. 1979 Oct;38(4):734–739. doi: 10.1128/aem.38.4.734-739.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks C. W. Sorption of heterotrophic and enteric bacteria to glass surfaces in the continuous culture of river water. Appl Microbiol. 1974 Oct;28(4):572–578. doi: 10.1128/am.28.4.572-578.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb S., Norman R. S. Osmotic power plants. Science. 1975 Aug 22;189(4203):654–655. doi: 10.1126/science.189.4203.654. [DOI] [PubMed] [Google Scholar]
- Marszalek D. S., Gerchakov S. M., Udey L. R. Influence of substrate composition on marine microfouling. Appl Environ Microbiol. 1979 Nov;38(5):987–995. doi: 10.1128/aem.38.5.987-995.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickels J. S., Bobbie R. J., Lott D. F., Martz R. F., Benson P. H., White D. C. Effect of manual brush cleaning on biomass and community structure of microfouling film formed on aluminum and titanium surfaces exposed to rapidly flowing seawater. Appl Environ Microbiol. 1981 Jun;41(6):1442–1453. doi: 10.1128/aem.41.6.1442-1453.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen K. Method for studying microbial biofilms in flowing-water systems. Appl Environ Microbiol. 1982 Jan;43(1):6–13. doi: 10.1128/aem.43.1.6-13.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]



