Abstract
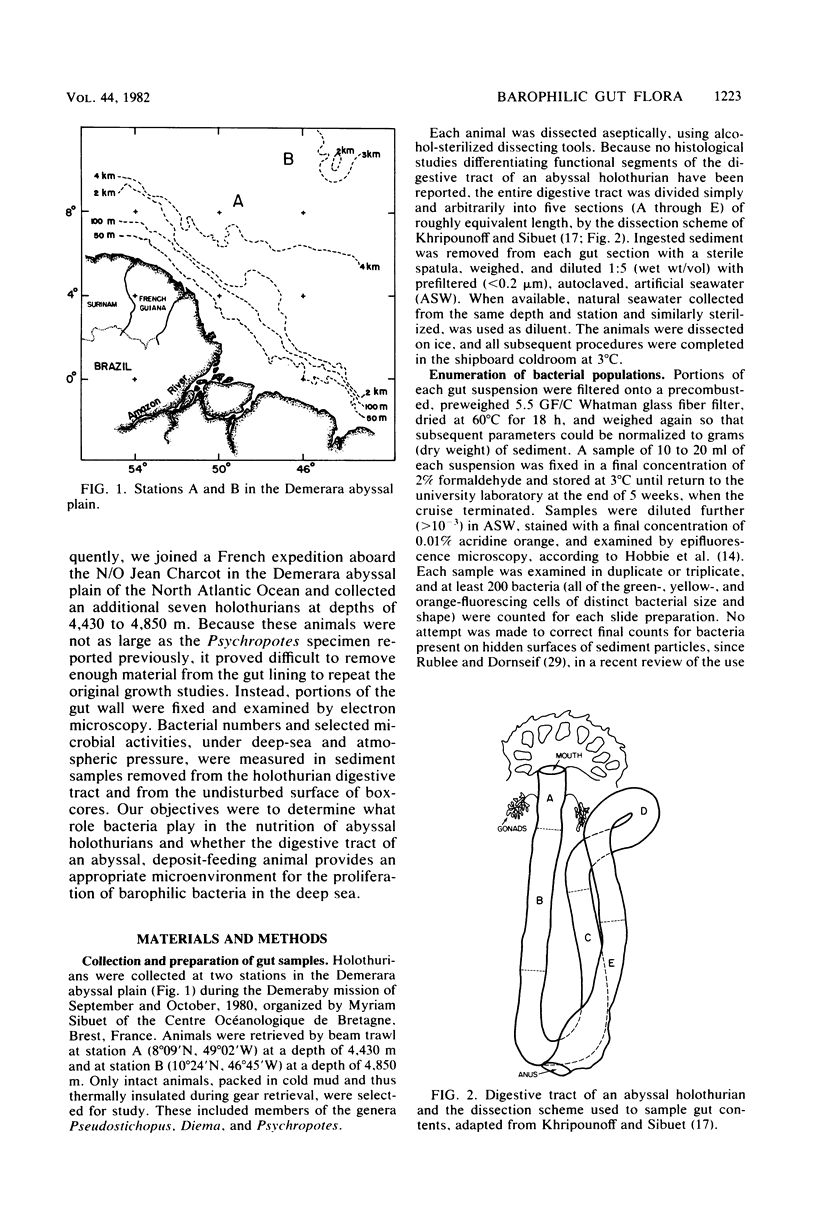
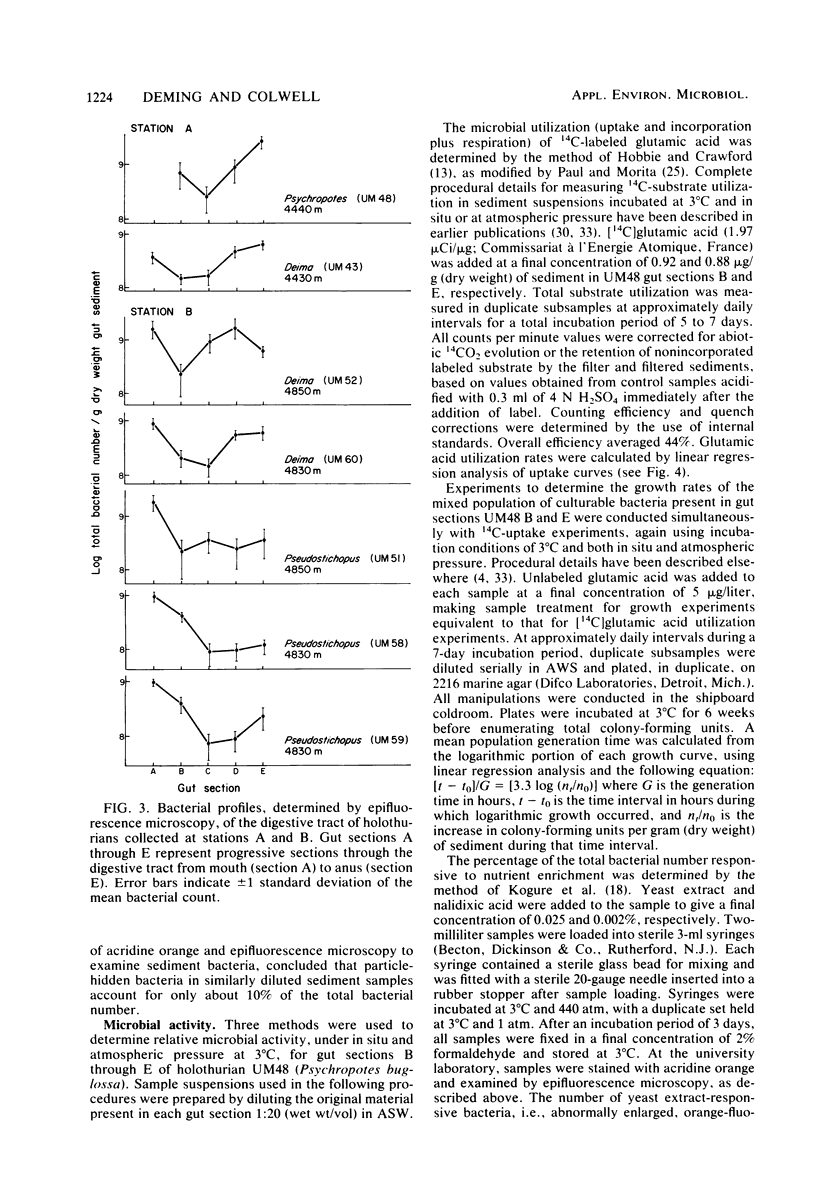
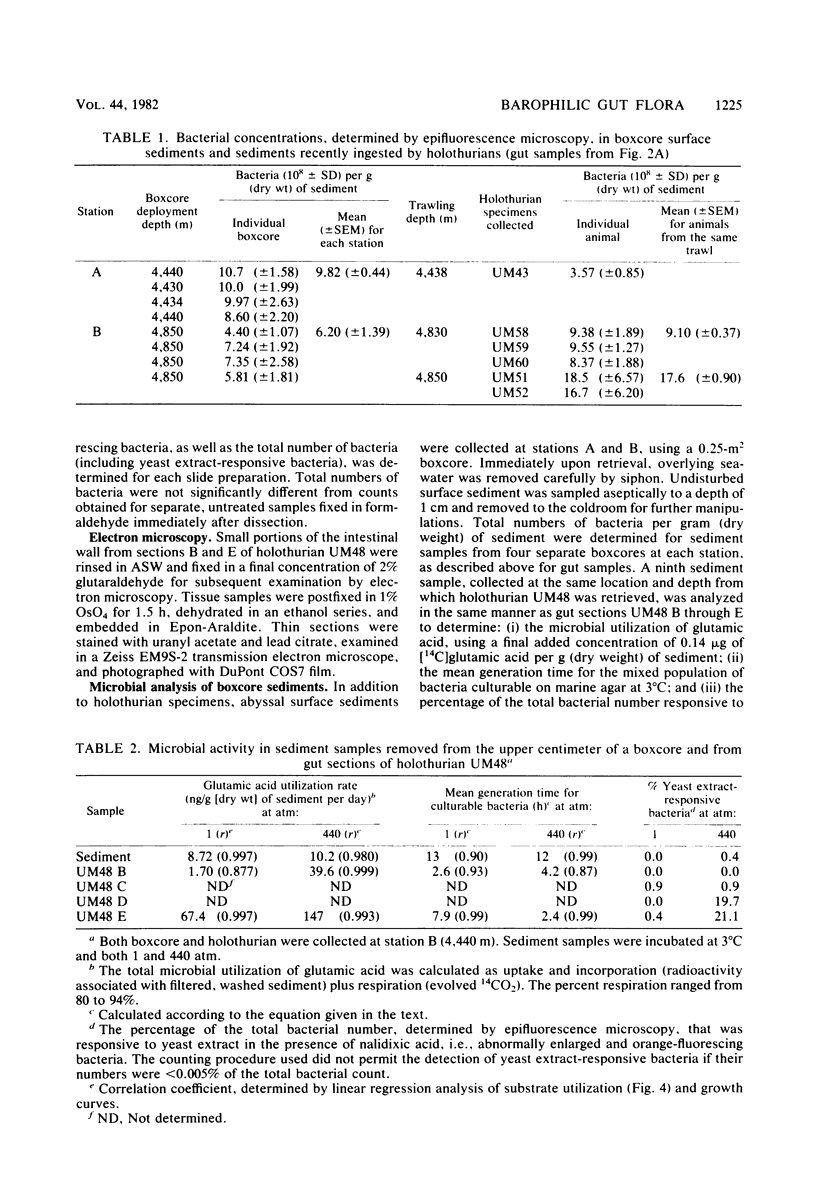
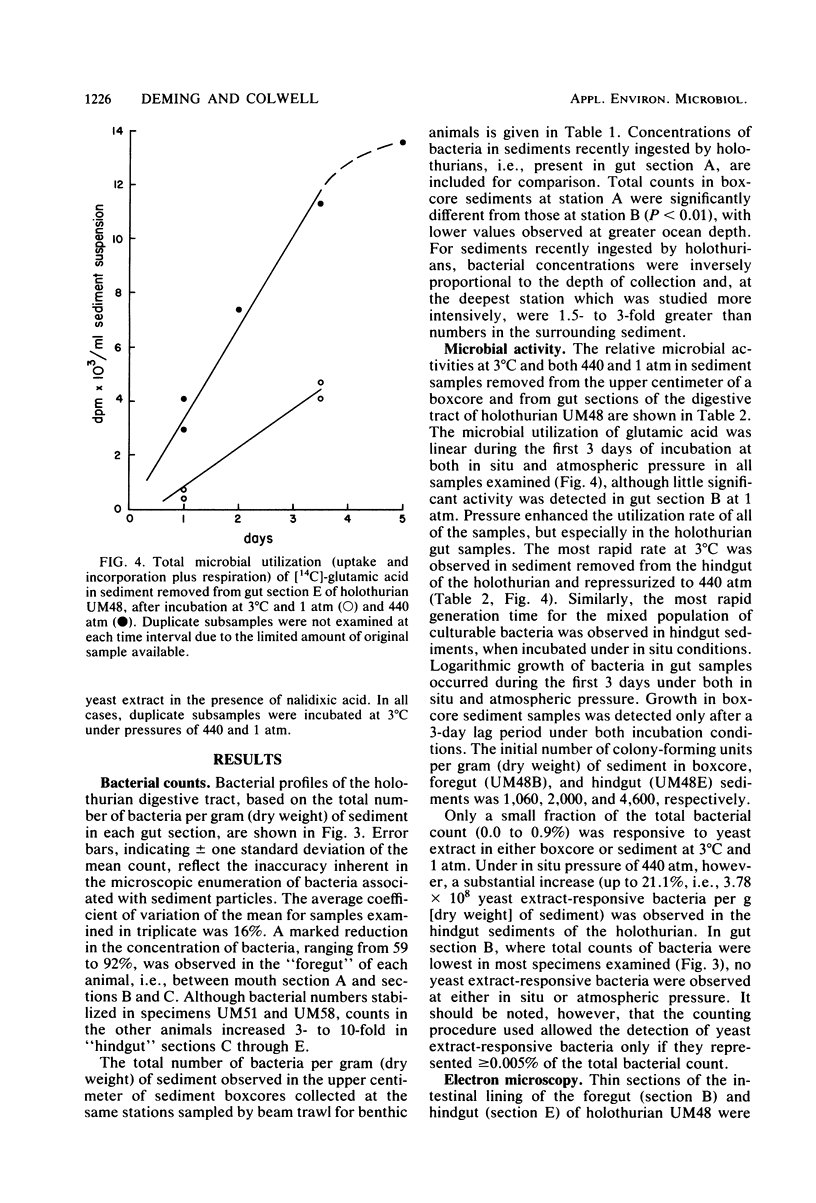
Abyssal holothurians and sediment samples were collected at depths of 4,430 to 4,850 m in the Demerara abyssal plain. Bacterial concentrations in progressive sections of the holothurian digestive tract, as well as in surrounding surface sediments, were determined by epifluorescence microscopy. Total bacterial counts in sediments recently ingested by the animals were 1.5- to 3-fold higher than in surrounding sediments at the deepest station. Lowest counts were observed consistently in the foregut, where the digestive processes of the holothurian are believed to occur. In most animals, counts increased 3- to 10-fold in the hindgut. Microbial activity at 3°C and in situ and atmospheric pressure were determined for gut and sediment samples by measuring the utilization of [14C]glutamic acid, the doubling time of the mixed-population of culturable bacteria, and the percentage of the total bacterial count responsive to yeast extract in the presence of nalidixic acid, using epifluorescence microscopy. A barophilic microbial population, showing elevated activity under deep-sea pressure, was detected by all three methods in sediments removed from the hindgut. Transmission electron micrographs revealed intact bacteria directly associated with the intestinal lining only in the hindgut. The bacteria are believed to be carried as an actively metabolizing, commensal gut flora that transforms organic matter present in abyssal sediments ingested by the holothurian. Using data obtained in this study, it was calculated that sediment containing organic matter altered by microbial activity cleared the holothurian gut every 16 h, suggesting that abyssal holothurians and their associated gut flora are important participants in nutrient cycles of the abyssal benthic ocean.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hessler R. R., Isaacs J. D., Mills E. L. Giant amphipod from the abyssal pacific ocean. Science. 1972 Feb 11;175(4022):636–637. doi: 10.1126/science.175.4022.636. [DOI] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogure K., Simidu U., Taga N. A tentative direct microscopic method for counting living marine bacteria. Can J Microbiol. 1979 Mar;25(3):415–420. doi: 10.1139/m79-063. [DOI] [PubMed] [Google Scholar]
- Morita R. Y. Microbial life in the deep sea. Can J Microbiol. 1980 Dec;26(12):1375–1385. doi: 10.1139/m80-230. [DOI] [PubMed] [Google Scholar]
- Paul K. L., Morita R. Y. Effects of hydrostatic pressure and temperature on the uptake and respiration of amino acids by a facultatively psychrophilic marine bacterium. J Bacteriol. 1971 Nov;108(2):835–843. doi: 10.1128/jb.108.2.835-843.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz J. R., Colwell R. R. Heterotrophic activity of deep-sea sediment bacteria. Appl Microbiol. 1975 Oct;30(4):639–649. doi: 10.1128/am.30.4.639-649.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz J. R., Yayanos A. A., Colwell R. R. Metabolic activities of the intestinal microflora of a deep-sea invertebrate. Appl Environ Microbiol. 1976 Jan;31(1):46–48. doi: 10.1128/aem.31.1.46-48.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor P. S., Deming J. W., Ohwada K., Colwell R. R. Activity and growth of microbial populations in pressurized deep-sea sediment and animal gut samples. Appl Environ Microbiol. 1982 Aug;44(2):413–422. doi: 10.1128/aem.44.2.413-422.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. M., Carlucci A. F. Bacterial utilisation of organic matter in the deep sea. Nature. 1976 Aug 26;262(5571):810–811. doi: 10.1038/262810a0. [DOI] [PubMed] [Google Scholar]
- Yayanos A. A., Dietz A. S., VAN Boxtel R. Isolation of a deep-sea barophilic bacterium and some of its growth characteristics. Science. 1979 Aug 24;205(4408):808–810. doi: 10.1126/science.205.4408.808. [DOI] [PubMed] [Google Scholar]
- Yayanos A. A. Recovery and maintenance of live amphipods at a pressure of 580 bars from an ocean depth of 5700 meters. Science. 1978 Jun 2;200(4345):1056–1059. doi: 10.1126/science.200.4345.1056. [DOI] [PubMed] [Google Scholar]
- ZOBELL C. E., MORITA R. Y. Barophilic bacteria in some deep sea sediments. J Bacteriol. 1957 Apr;73(4):563–568. doi: 10.1128/jb.73.4.563-568.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]