Abstract
A strain of the starch-converting yeast Lipomyces kononenkoae produced, when grown on starch, a debranching enzyme that proved to be an isoamylase (glycogen 6-glucanohydrolase; E.C. 3.2.1.68). So far, only bacteria have been found to produce extracellular isoamylases. The yeast isoamylase enhanced β-amylolysis of amylopectin and glycogen and completely hydrolyzed these substrates into maltose when combined with a β-amylase but had no action on dextran or pullulan. By isopropanol precipitation and carboxymethyl cellulose chromatography, L. kononenkoae isoamylase was partially purified from the supernatant of cultures grown on a mineral medium with soluble starch. Optimum temperature and pH for activity of the isoamylase were 30°C and 5.6. The molecular weight was around 65,000, and the pI was at pH 4.7 to 4.8. The Km (30°C, pH 5.5) for soluble starch was 9 g liter−1.
Full text
PDF
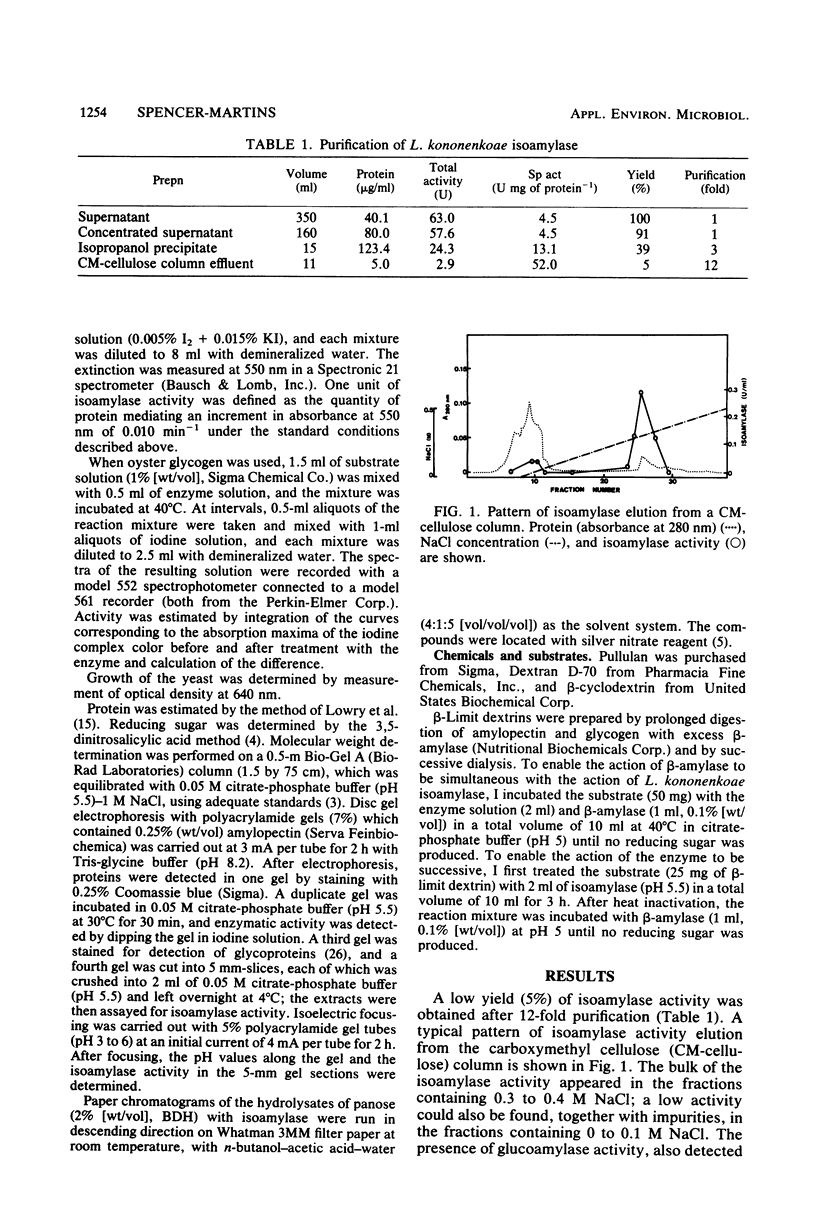
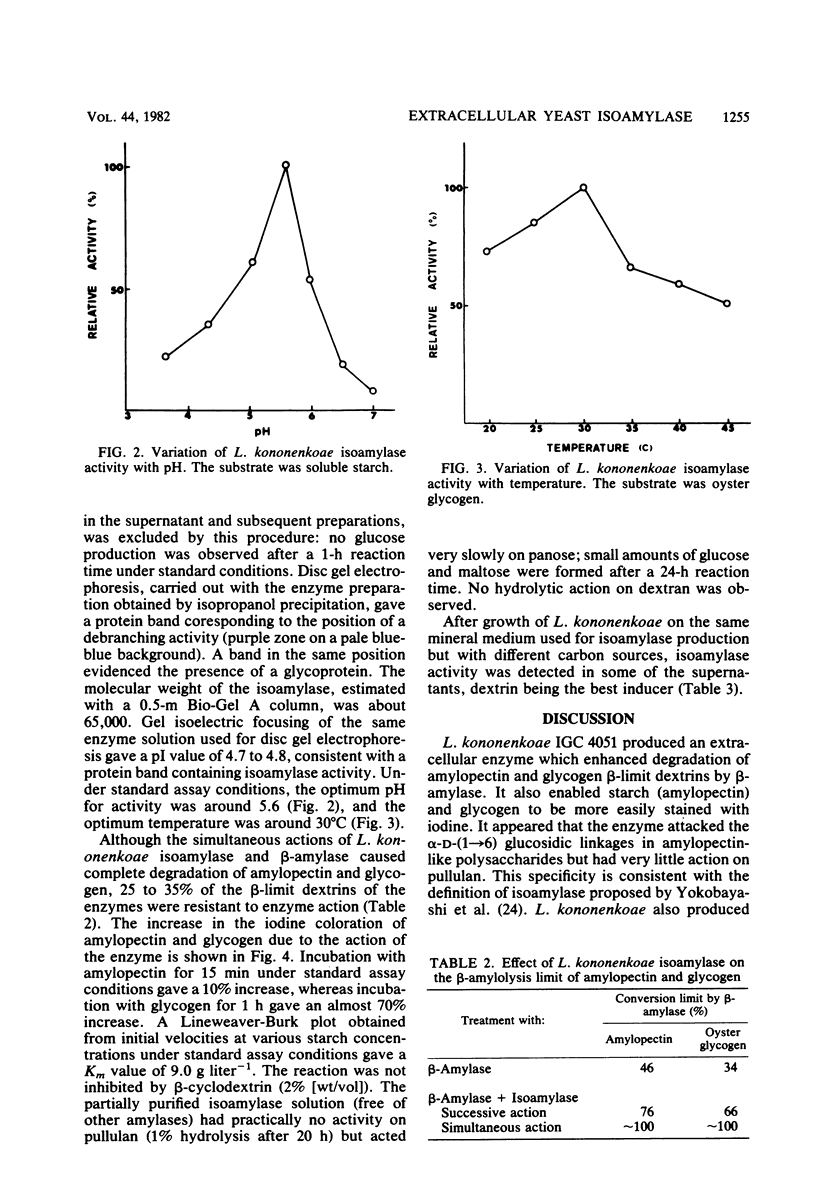


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amemura A., Konishi Y., Harada T. Molecular weight of the undegraded polypeptide chain of Pseudomonas amyloderamosa isoamylase. Biochim Biophys Acta. 1980 Feb 14;611(2):390–393. doi: 10.1016/0005-2744(80)90077-7. [DOI] [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunja-Smith Z., Marshall J. J., Smith E. E., Whelan W. J. A glycogen-debranching enzyme from Cytophaga. FEBS Lett. 1970 Dec 28;12(2):96–100. doi: 10.1016/0014-5793(70)80572-5. [DOI] [PubMed] [Google Scholar]
- Gunja Z. H., Manners D. J., Maung K. Studies on carbohydrate-metabolizing enzymes. 7. Yeast isoamylase. Biochem J. 1961 Nov;81(2):392–398. doi: 10.1042/bj0810392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T., Yokobayashi K., Misaki A. Formation of isoamylase by Pseudomonas. Appl Microbiol. 1968 Oct;16(10):1439–1444. doi: 10.1128/am.16.10.1439-1444.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iurkevich V. V., Shurygina N. N. Mekhanizm vliianiia krakhmala na biosintez al'fa-amilazy Aspergillus oryzae. Prikl Biokhim Mikrobiol. 1972 Sep-Oct;8(5):515–519. [PubMed] [Google Scholar]
- Jeanningros R., Creuzet N., Frixon C., Cattaneo J. Proceedings: A debranching enzyme in Escherichia coli. Biochem Soc Trans. 1975;3(2):336–337. doi: 10.1042/bst0030336. [DOI] [PubMed] [Google Scholar]
- KJOLBERG O., MANNERS D. J. Studies on carbohydrate-metabolizing enzymes. 9. The action of isoamylase on amylose. Biochem J. 1963 Feb;86:258–262. doi: 10.1042/bj0860258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kainuma K., Kobayashi S., Harada T. Action of Pseudomonas isoamylase on various branched oligo and poly-saccharides. Carbohydr Res. 1978 Mar;61:345–357. doi: 10.1016/s0008-6215(00)84494-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee E. Y., Carter J. H., Nielsen L. D., Fischer E. H. Purification and properties of yeast amylo-1,6-glucosidase--oligo-1,4 leads to 1,4-glucantransferase. Biochemistry. 1970 May 26;9(11):2347–2355. doi: 10.1021/bi00813a019. [DOI] [PubMed] [Google Scholar]
- MARUO B., KOBAYASHI T. Enzymic scission of the branch links of amylopectin. Nature. 1951 Apr 14;167(4250):606–607. doi: 10.1038/167606a0. [DOI] [PubMed] [Google Scholar]
- Marshall J. J. Inhibition of pullulanase by Schardinger dextrins. FEBS Lett. 1973 Dec 1;37(2):269–273. doi: 10.1016/0014-5793(73)80476-4. [DOI] [PubMed] [Google Scholar]
- Ueda S., Nanri N. Production of isoamylase by Escherichia intermedia. Appl Microbiol. 1967 May;15(3):492–496. doi: 10.1128/am.15.3.492-496.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokobayashi K., Akai H., Sugimoto T., Hirao M., Sugimoto K., Harada T. Comparison of the kinetic parameters of Pseudomonas isoamylase and Aerobacter pullulanase. Biochim Biophys Acta. 1973 Jan 12;293(1):197–202. doi: 10.1016/0005-2744(73)90391-4. [DOI] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]
- van Uden N. Transport-limited fermentation and growth of saccharomyces cerevisiae and its competitive inhibition. Arch Mikrobiol. 1967;58(2):155–168. doi: 10.1007/BF00406676. [DOI] [PubMed] [Google Scholar]


