Abstract
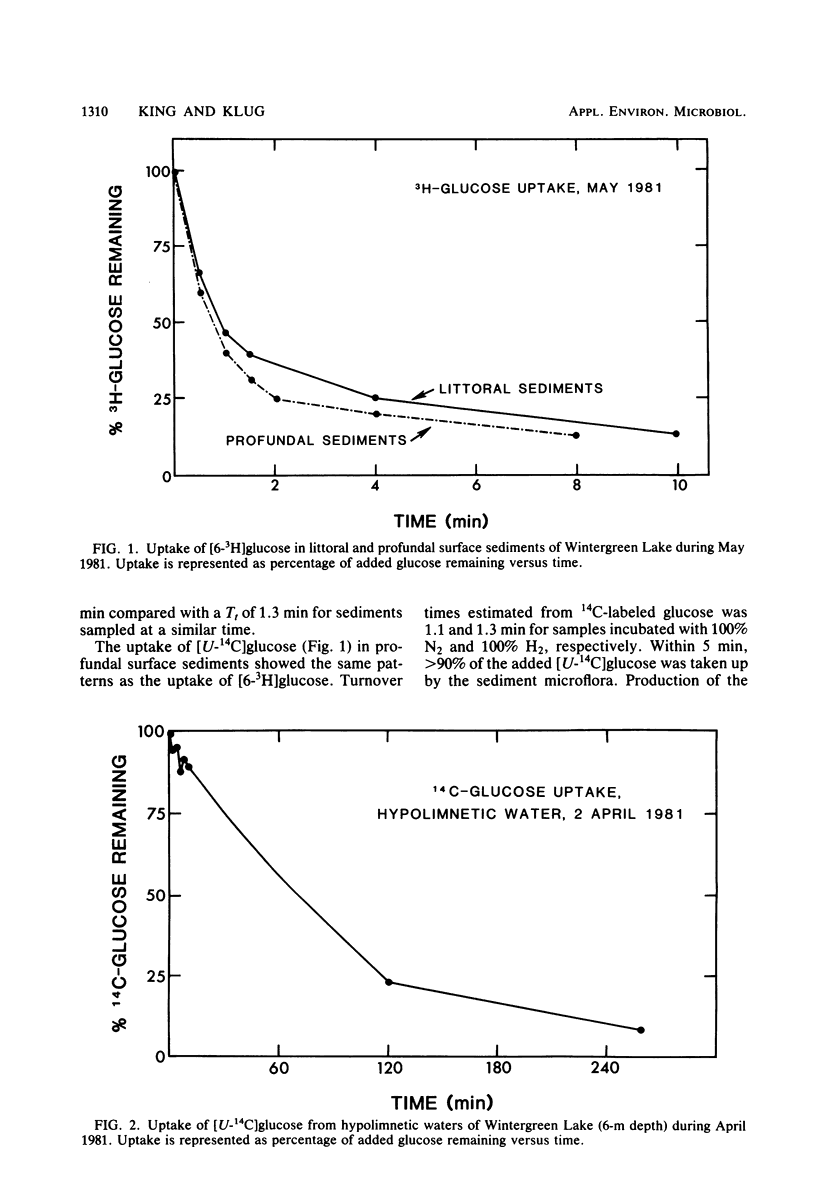
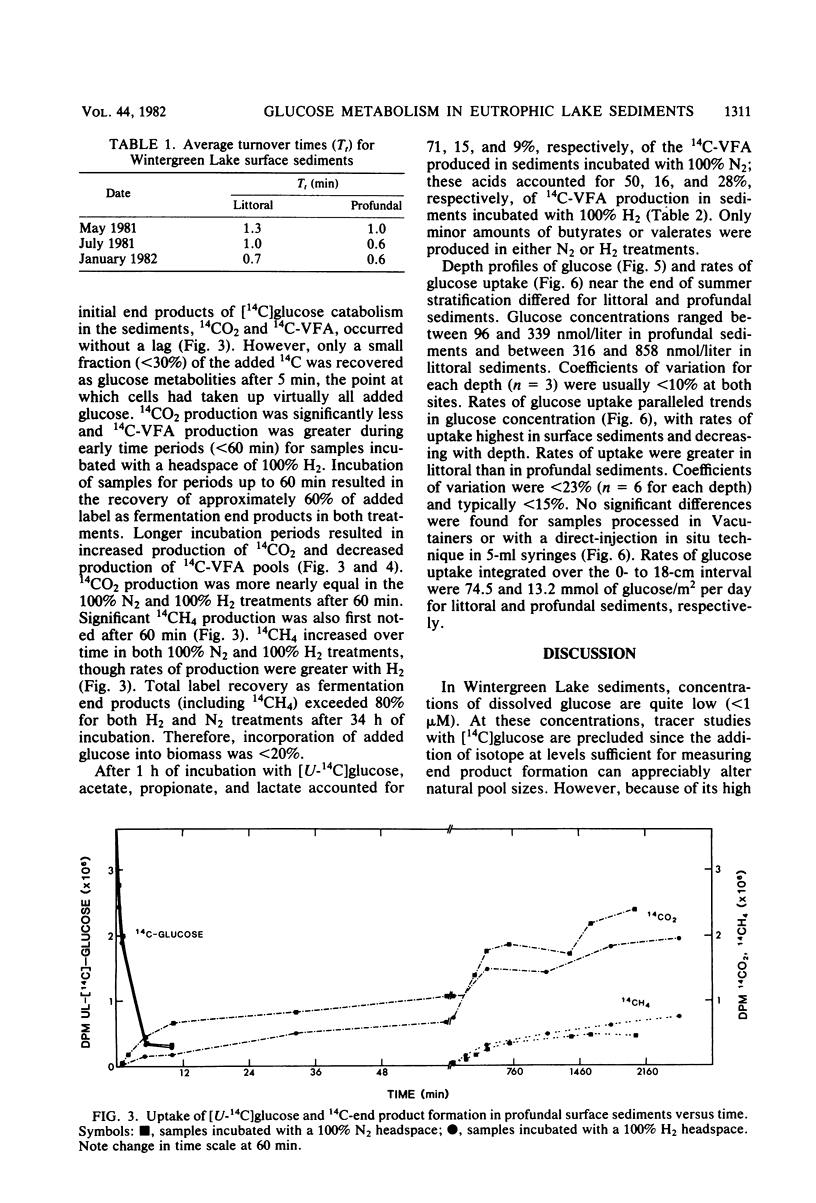
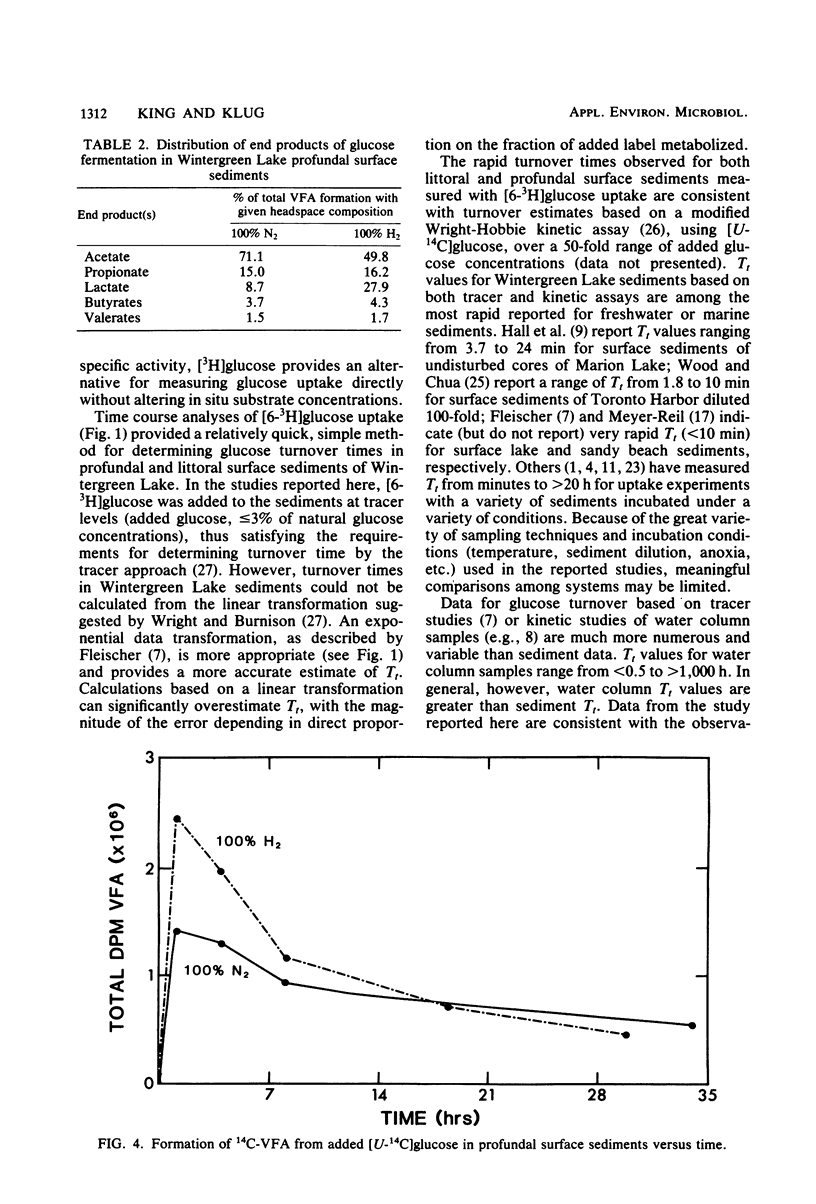
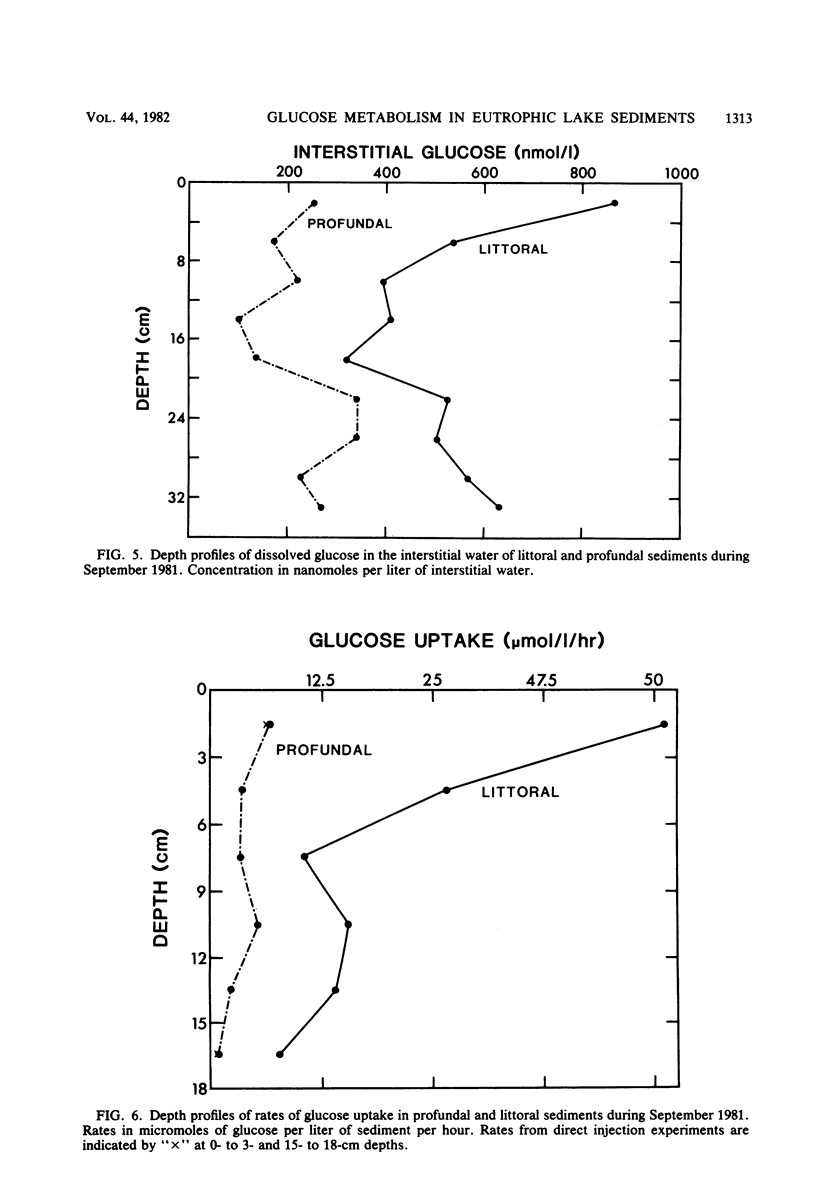
The uptake of glucose and the formation of end products from glucose catabolism have been measured for sediments of eutrophic Wintergreen Lake with a combination of tritiated and 14C-labeled tracers. Time course analyses of the loss of [3H]glucose from sediments were used to establish rate constants for glucose uptake at natural substrate concentrations. Turnover times from these analyses were about 1 min for littoral and profundal sediments. No seasonal or site differences were noted in turnover times. Time course analyses of [U-14C]glucose uptake and 14C-labeled end product formation indicated that glucose mass flow could not be calculated from end product formation since the specific activity of added [14C]glucose was significantly diluted by pools of intracellular glucose and glucose metabolites. Mass flow could only be accurately estimated by use of rates of uptake from tracer studies. Intermediate fermentation end products included acetate (71%), propionate (15%), lactate (9%), and only minor amounts of butyrates or valerates. Addition of H2 to sediments resulted in greater production of lactate (28%) and decreased formation of acetate (50%), but did not affect glucose turnover. Depth profiles of glucose uptake indicated that rates of uptake decreased with depth over the 0- to 18-cm interval and that glucose uptake accounted for 30 to 40% of methanogenesis in profundal sediments.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chung K. T. Inhibitory effects of H2 on growth of Clostridium cellobioparum. Appl Environ Microbiol. 1976 Mar;31(3):342–348. doi: 10.1128/aem.31.3.342-348.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison M. J., Wright R. T., Morita R. Y. Method for measuring mineralization in lake sediments. Appl Microbiol. 1971 Apr;21(4):698–702. doi: 10.1128/am.21.4.698-702.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. M., Klug M. J. Comparative aspects of sulfur mineralization in sediments of a eutrophic lake basin. Appl Environ Microbiol. 1982 Jun;43(6):1406–1412. doi: 10.1128/aem.43.6.1406-1412.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Klug M. J. Intermediary metabolism of organic matter in the sediments of a eutrophic lake. Appl Environ Microbiol. 1982 Mar;43(3):552–560. doi: 10.1128/aem.43.3.552-560.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. L., Klug M. J. Reduction of sulfur compounds in the sediments of a eutrophic lake basin. Appl Environ Microbiol. 1981 May;41(5):1230–1237. doi: 10.1128/aem.41.5.1230-1237.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toerien D. F., Cavari B. Effect of temperature on heterotrophic glucose uptake, mineralization, and turnover rates in lake sediments. Appl Environ Microbiol. 1982 Jan;43(1):1–5. doi: 10.1128/aem.43.1.1-5.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer P. J., Zeikus J. G. Fermentation of cellulose and cellobiose by Clostridium thermocellum in the absence of Methanobacterium thermoautotrophicum. Appl Environ Microbiol. 1977 Feb;33(2):289–297. doi: 10.1128/aem.33.2.289-297.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood L. W., Chua K. E. Glucose flux at the sediment-water interface of Toronto Harbour, Lake Ontario, with reference to pollution stress. Can J Microbiol. 1973 Apr;19(4):413–420. doi: 10.1139/m73-069. [DOI] [PubMed] [Google Scholar]



