Abstract
Microbial carbamoyl phosphate synthetases (CPS) use glutamine as nitrogen donor and are composed of two subunits (or domains), one exhibiting glutaminase activity, the other able to synthesize carbamoyl phosphate (CP) from bicarbonate, ATP, and ammonia. The pseudodimeric organization of this synthetase suggested that it has evolved by duplication of a smaller kinase, possibly a carbamate kinase (CK). In contrast to other prokaryotes the hyperthermophilic archaeon Pyrococcus furiosus was found to synthesize CP by using ammonia and not glutamine. We have purified the cognate enzyme and found it to be a dimer of two identical subunits of Mr 32,000. Its thermostability is considerable, 50% activity being retained after 1 h at 100°C or 3 h at 95°C. The corresponding gene was cloned by PCR and found to present about 50% amino acid identity with known CKs. The stoichiometry of the reaction (two ATP consumed per CP synthesized) and the ability of the enzyme to catalyze at high rate a bicarbonate-dependent ATPase reaction however clearly distinguish P. furiosus CPS from ordinary CKs. Thus the CPS of P. furiosus could represent a primeval step in the evolution of CPS from CK. Our results suggest that the first event in this evolution was the emergence of a primeval synthetase composed of subunits able to synthesize both carboxyphosphate and CP; this step would have preceded the duplication assumed to have generated the two subdomains of modern CPSs. The gene coding for this CK-like CPS was called cpkA.
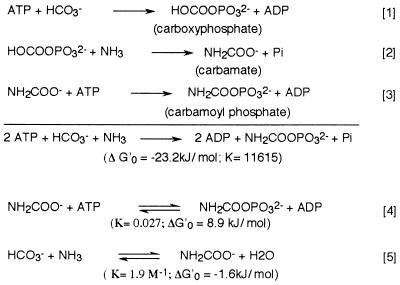
Carbamoyl phosphate (CP) is a key metabolite in nitrogen metabolism because it is a precursor of both arginine and the pyrimidines. Three classes of carbamoyl phosphate synthetases (CPS, EC 6.3.5.5) have been recognized on the basis of their substrate specificity—whether glutamine or ammonia is used as the nitrogen donor—and their requirement for N-acetyl-l-glutamate as allosteric effector (reviewed in refs. 1 and 2). Despite this apparent diversity, all three forms of CPS consist of two entities, a glutaminase domain (or subunit) and a synthetase domain (or subunit) that catalyzes ATP-dependent synthesis of CP from ammonia and bicarbonate. In prokaryotic and fungal arginine-specific CPSs the two functions are fulfilled by separate polypeptides, whereas in other organisms the homologous parts of the molecule are domains within the same polypeptide. The synthesis of CP by the synthetase domain or subunit of CPS proceeds in three consecutive steps (1, 3, 4); two ATP molecules are consumed in the process (Fig. 1).
Figure 1.
Reactions catalyzed by CPS (1–3), by CK (4), and chemical equilibrium of carbamate with bicarbonate and ammonia (5).
Analysis of the DNA sequence encoding the synthetase subunit of Escherichia coli (the carB gene) revealed considerable similarity between the N- and C-terminal halves of the protein, and it was proposed that the synthetase gene had evolved by duplication of a smaller ancestral gene—possibly coding for a carbamate kinase (CK) (5). CKs (EC 2.7.2.2) are homodimers composed of subunits of 31–37 kDa (6). As carbamate is in chemical equilibrium with bicarbonate and ammonia, CK can synthesize CP from HCO3−, NH3 and one molecule of ATP (Fig. 1). This evolutionary scheme found support in the weak but nevertheless suggestive sequence similarities that were brought to light between carB and the gene for CK in Pseudomonas aeruginosa (7). Moreover, further studies showed that the N-terminal half of the synthetase (subdomain CPS.A) catalyzed the synthesis of carboxyphosphate (Fig. 1, reaction 1), whereas the C-terminal part was responsible for the second ATP-dependent reaction, the synthesis of CP (Fig. 1, reaction 3) (8–11). This evidence supports the suggestion that each one of the copies of the ancestral kinase has become specialized in one of the two ATP-dependent reactions involved in CP synthesis (12–14).
We have reported a thermostable CPS activity in the hyperthermophile archaeon Pyrococcus furiosus. This activity appears unique among the microorganisms investigated so far in that ammonia and not glutamine is the nitrogen donor of the reaction (15). The enzyme appears to channel the very thermolabile CP molecule toward citrulline, presumably by associating with ornithine transcarbamylase (OTC) in a multienzyme complex (15). In the present study, the enzyme was purified 350-fold and the protein studied in detail. The corresponding gene was cloned and its sequence determined. Whereas the genetic data reveal substantial similarities with CKs, several properties of the enzyme—such as the stoichiometry of the reaction and its ability to catalyze a high-rate, bicarbonate-dependent ATPase reaction—clearly distinguish it from ordinary CKs.
The data support the hypothesis that P. furiosus CPS indeed is related to the ancestral form of the synthetase component of CPSs; however, they also suggest that the first step in the evolution from an ancestral CK toward CPS consisted of mutations conferring to the CK subunit the capacity to catalyze either one of the two ATP-dependent reactions.
MATERIALS AND METHODS
Chemicals.
P1.P3 di-(adenosine-5′)-triphosphate (Ap3A), P1.P5 di-(adenosine-5′)-pentaphosphate (Ap5A), and α,β methylene-ATP were from Sigma. Na214CO3 was from Amersham. The Original TA Cloning Kit was from Invitrogen. Purified CPS of E. coli was from this laboratory (courtesy of S. Delannay).
Bacterial Strain and Culture Conditions.
P. furiosus strain Vc1 (DSM 3638) (16) was grown at 95°C under anaerobic conditions as described (15). Streptococcus faecalis strain ATCC 11700 was grown on complex medium with 0.5% arginine and 2.5% glucose.
CPS Assay.
Activity was measured as previously described (15) except that MgATP concentration was 3 mM instead of 10 mM. One enzyme unit is defined as the amount of enzyme that converts 1 μmol substrate to product h−1.
Determination of Protein.
Protein was measured by the Lowry method (17), or in the last purification steps by UV absorption.
Stoichiometry of the CPS Reaction.
The stoichiometry of the reaction was determined by comparing the rate of ATP consumption and of CP production. The rate of ATP consumption was measured at 37°C by using the coupled assay with pyruvate kinase and lactate dehydrogenase described by Miran et al. (18). The reaction mixture contained in a volume of 1.0 ml, 100 mM Tris⋅HCl (pH 8), 1 mM phosphoenolpyruvate, 20 units of pyruvate kinase, 30 units of lactate dehydrogenase, 200 mM NH4Cl, 40 mM NaHCO3, 0.8 mM NADH, 3 mM MgATP, and partially purified CPS enzyme. The disappearance of NADH was monitored by following the absorbance at 340 nm. The rate of CP production was followed with the coupled CPS–OTC assay (15).
Enzyme Purification.
Frozen cells (50 g wet mass) were thawed in 50 ml of 50 mM Tris⋅HCl buffer (pH 7.2) supplemented with DNase (10 μg/ml) and disrupted by sonication for 20 min in a Raytheon sonic oscillator (250 W, 10 kHz); the suspension was centrifuged at 80,000 × g for 30 min. Solid ammonium sulfate was added to 40% saturation and stirred for 30 min. The solution was then centrifuged at 12,000 × g for 20 min. The supernatant fluid was brought to 80% saturation, stirred for 30 min, and centrifuged as described above. The pellet was suspended in 50 mM Tris⋅HCl buffer (pH 7.2), dialyzed against the same buffer, and applied on a DEAE Sepharose CL6B 26/40 column equilibrated with 50 mM Tris⋅HCl buffer (pH 7.2). The CPS activity was eluted with a linear gradient of 0–0.5 M KCl in 50 mM Tris⋅HCl buffer (pH 7.2). The active fractions were pooled, concentrated, dialyzed against 50 mM Tris⋅HCl buffer (pH 7.2), and applied on a Blue Sepharose 16/16 column equilibrated with 20 mM Tris⋅HCl buffer (pH 7.2) and 5 mM MgCl2. The column was washed with 20 mM Tris⋅HCl buffer (pH 7.2) and 5 mM MgCl2. The CPS activity was eluted with 50 mM Tris⋅HCl buffer (pH 7.2) and 20 mM MgATP. The active fractions were collected, concentrated and applied on a Sephadex G200 26/40 column equilibrated with 50 mM Tris⋅HCl buffer (pH 7.2). Active fractions were collected and applied to a Superose P12 HR 10/30 (fast protein liquid chromatography, FPLC) equilibrated with 50 mM Tris⋅HCl buffer (pH 7.2). For the last step, active fractions were applied to a Mono-Q HR 5/5 column (FPLC) equilibrated with 50 mM Tris⋅HCl buffer (pH 7.2) and eluted with a linear gradient of KCl 0–0.5 M in 50 mM Tris⋅HCl.
Electrophoresis.
Electrophoresis under native and denaturing conditions (SDS) was performed on 8–25% gradient gels in the PHAST System (Pharmacia). Protein bands were visualized by staining with Coomassie brilliant blue.
Cloning of the CK-Like CPS Gene.
Cloning was achieved by PCR with Taq polymerase (15 sec at 94°C, 60 sec at 48°C, 30 sec at 72°C, 30 cycles) on 1 μg of genomic DNA of P. furiosus restricted by PstI and primed with 50 pmol of two degenerate oligonucleotides (GIBCO/BRL): 5′-CAY GGN AAY GGN CCH CA-3′ and 5′-TCY TTR TCD ATN ACD GC-3′ [n = A + C + T + G, r = A + G, Y = C + T, H = A + T + C, D = A + T + G] corresponding to two conserved regions of CK genes (see Fig. 3). A first fragment of 501 bp related to the middle of the gene was cloned into the TA cloning kit vector. The flanking regions of the gene were determined by inverse PCR as described by Eeles and Stamps (19). Genomic DNA (20 ng) was restricted separately by HindIII, ClaI, ScaI, or SacI and self-ligated. PCR was performed on each of the different circularized DNAs (30 sec at 94°C, 90 sec at 48°C, 90 sec at 72°C, 40 cycles) with 50 pmol of oligonucleotides: 5′-CTGCCCAGCATCCATATG-3′ and 5′-TCCTGTAATACTTGAAG-3′ (Fig. 3).
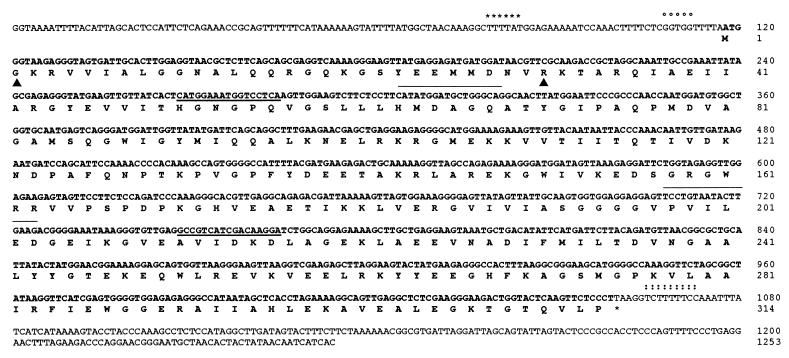
Figure 3.
Nucleotides and deduced amino acid sequence of the P. furiosus CPS. Numbering begins at the first nucleotide of the sequence. The A-box motif is indicated by stars (✽), B-box motif by circle (○), and termination site by double points (:). Upstream and downstream primers used for degenerated PCR are underlined; upstream and downstream primers used for inverse PCR are overlined. Amino acid sequence determined by Edman degradation is flanked by two triangles.
RESULTS
Enzyme Purification and Molecular Weight Determination.
CPS was purified 350-fold from frozen P. furiosus cells with a final yield of 6% (Table 1). The specific activity of the final preparation was 700 units/mg (measured at 60°C). Polyacrylamide gel electrophoresis in native conditions of the purified CPS revealed one major band at 80 kDa, in agreement with the Mr of 78,000 estimated on Superose P12 (Table 1). In SDS/PAGE a major and a minor band were observed. Amino-terminal sequence analysis of the major band of 32 kDa revealed 33–53% identity with the proximal part of known CKs (see Fig. 4), whereas the minor band, of higher Mr, revealed 61% similarity with phosphoglycerate dehydrogenase from Methanococcus jannaschii (20). P. furiosus CPS thus appears to be a dimer of two identical subunits of 32 kDa.
Table 1.
Purification of the CPS from P. furiosus
| Purification step | Total protein, mg | Total activity, units | Specific activity, units/mg | Yield, % | Purification rate |
|---|---|---|---|---|---|
| Crude extract | 4,400 | 8,500 | 2 | 100 | 1 |
| (NH4)2SO4 fractionation | 3,400 | 8,000 | 2.5 | 94 | 1.25 |
| DEAE-Sepharose | 800 | 8,300 | 10 | 97 | 5 |
| Blue Sepharose | 150 | 8,200 | 55 | 96 | 30 |
| Sephadex G200 | 20 | 4,400 | 220 | 52 | 110 |
| Superose P12 | 3.5 | 2,100 | 600 | 25 | 300 |
| Mono-Q | 0.75 | 500 | 700 | 6 | 350 |
Figure 4.
Multiple alignment of amino acid sequences (species code and GenBank accession numbers in parentheses) of the CPS from P. furiosus (Pyrfu; Y09829) and CK from Pseudomonas aeruginosa (Pseae; X14693), Clostridium perfringens (Clope; X97768), Halobacterium salinarium (Halsa; X80931), Synechocystis sp (Synec; D90917), and Hemophilus influenzae (Hemin; U32741). Identical residues are marked with asterisks, and conservative substitutions are marked with points. Amino acid sequence determined by Edman degradation is flanked by two triangles.
pH Dependence.
CP synthesis was measured as a function of pH in the presence of Tris⋅HCl buffer (200 mM) at 37°C and 60°C. The influence of pH was tested for values superior to 7 and incubation times were reduced to 10 min at 37°C and 5 min at 60°C, thus preserving the initial level of NaHCO3. At 37°C maximal activity was obtained between 7.8 and 8 and at 60°C for a value near pH 7.6.
Thermal Stability of P. furiosus CPS.
P. furiosus CPS was incubated at 95°C and at 100°C in phosphate buffer pH 7.5 (100 mM) and the residual activity was measured at 60°C. Fifty percent activity was retained after 3-h incubation at 95°C and 1-h incubation at 100°C.
Stoichiometry of the Reaction Catalyzed.
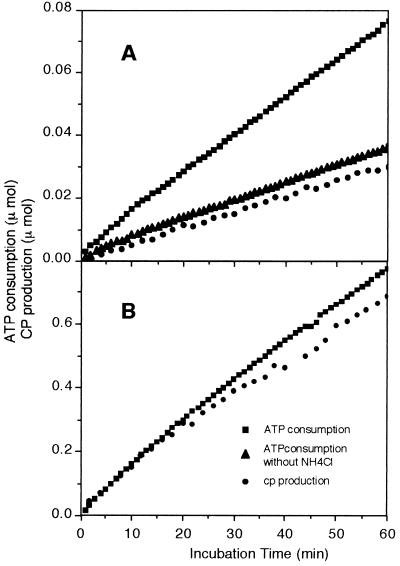
Per molecule of CP synthesized, the reaction catalyzed by CPS uses two ATP molecules, whereas the reaction catalyzed by CK uses only one (see Introduction). In the presence of ammonium chloride, CP synthesis catalyzed by P. furiosus CPS consumed two ATP molecules per molecule of CP formed (Fig. 2A). In the absence of ammonium chloride, the enzyme catalyzed a bicarbonate-dependent ATPase reaction consuming half the amount of ATP, as does E. coli CPS (21). This result indicates that the complete set of reactions catalyzed by the P. furiosus enzyme includes a step of bicarbonate phosphorylation. As a control we performed the stoichiometry test with S. faecalis CK (Fig. 2B); as expected, only one molecule of ATP was consumed per molecule of CP synthesized.
Figure 2.
Stoichiometry of ATP consumption and CP production in the reaction catalyzed by purified P. furiosus CPS (A) and S. faecalis CK (B).
Inhibition by Ap3A and Ap5A.
Ap5A and Ap3A are composed of two adenosyl groups ligated by five and three phosphates, respectively. CPS was inhibited by Ap5A, providing evidence that it has two separate binding sites for ATP (22). Moreover, it was not inhibited by Ap3A, suggesting that the distance between the two sites determines the sensitivity to compounds of the ApnA type (22). To our knowledge the effect of the compounds on CK activity has not yet been tested.
CP production by purified P. furiosus and E. coli CPSs, as well as by S. faecalis CK were tested at 37°C in the presence of inhibitor and at a concentration of ATP equal either to their respective S0.5 values or to 20% of this value. The amount of CP formed was determined by using the coupled assay with pyruvate kinase and lactate dehydrogenase or by using the coupled assay with OTC. The results obtained were identical with the two methods. When tested at an ATP concentration equal to 20% of the S0.5 (60 and 300 μM respectively), Ap3A up to the 1 mM range had no effect on P. furiosus and E. coli CPS. By contrast, 1 mM Ap5A exerted 74.4% inhibition on the P. furiosus enzyme, and 84.3% on E. coli CPS (assayed with glutamine). When tested at a concentration of ATP equal to S0.5, the inhibition was 25% weaker for both P. furiosus and E. coli CPS incubated with glutamine as nitrogen donor; 47% less inhibition was observed for E. coli CPS incubated with NH4Cl as nitrogen donor. CP synthesis by CK of S. faecalis was inhibited 71.5% by 1 mM Ap5A and 16% by 1 mM Ap3A.
Inhibition by α,β-Methylene-ATP.
α,β-Methylene-ATP is an analogue of ATP that inhibits the bicarbonate-dependent ATPase activity (Fig. 1, reaction 1) of CPS (23). At 37°C and at a concentration of ATP equal to half the respective S0.5 values of the cognate enzymes (15), 5 mM α,β- methylene ATP strongly inhibited CP synthesis by both E. coli and P. furiosus enzymes (99.7% and 91.6%, respectively). This result corroborates the stoichiometry test which already indicated that the catalytic process of the P. furiosus enzyme proceeds through a step of bicarbonate phosphorylation.
Regulatory Properties.
The influence of a series of nucleotides on enzyme activity was measured at 37°C, 60°C, and 90°C at a concentration of 1 mM in the presence of 0.3 mM MgATP. AMP was the only nucleoside monophosphate to inhibit (about 39%). Among the nucleoside triphosphates, ITP and CTP had a small inhibitory effect (44% and 31%, respectively); GTP and UTP were not inhibitory. ADP inhibited strongly (93%), which may be related to the fact that it is a product of the reaction. The temperature did not influence these effects. No effect of ornithine was observed at any temperature.
Molecular Cloning and Sequence Analysis of the Gene Coding for the CPS of P. furiosus.
The sequence of the first 30 residues of purified P. furiosus CPS by Edman degradation revealed similarities to N-terminal sequences of CKs. Therefore, the gene coding for the CPS of P. furiosus was cloned by PCR using degenerated oligonucleotides based on the comparison of known CK genes. A fragment of 501 bp related to the middle of the gene was first cloned. The flanking regions of the gene were determined by inverse PCR (Fig. 3). The total sequence of the P. furiosus fragments cloned revealed a 941-bp ORF coding for a protein of 314 residues with a calculated Mr of 34,400, a finding in agreement with the value determined by SDS/PAGE. The sequence confirmed the Edman degradation data. The N-terminal methionine is absent from the protein. The deduced amino acid sequence showed 57% to 63% similarity and 49.2% to 50.9% identity with known CKs (Fig. 4). We propose to call this gene cpkA (GenBank accession no. Y09829) (see Discussion).
Six nucleotides upstream from the coding sequence there is a putative ribosome binding site (GGTG), itself preceded, between nucleotides −34 and −39, by an hexameric A+T-rich A-box motif similar to other archaeal promoters (24). The region located 6–10 nucleotides upstream of the ATG codon exhibits similarity with the less conserved B-box sequence 5′-T/CG/A-3′ (25). Downstream of the cpkA gene, the T+C rich region (3–11 bp after the stop codon) could constitute a termination site (26) (Fig. 3). The G+C content of cpkA is 44%, which is consistent with the GC content of the P. furiosus genome (16).
DISCUSSION
At an early stage of this study we had reported the presence of a thermostable CPS activity in extracts and partially purified preparations from the hyperthermophilic archaeon P. furiosus (15). An outstanding feature of the reaction was its dependence on ammonia rather than glutamine as a nitrogen donor, in contrast with the reaction catalyzed by the CPSs found in all microorganisms investigated up to now.
The corresponding enzyme was purified and shown to be a protein of Mr about 80,000; it is a homodimer of a subunit of Mr about 32,000; the gene itself was found to consist of a 941-bp-long ORF encoding 314 potential residues adding up to a calculated Mr of 34,400. The dimeric structure of the enzyme and its Mr are thus reminiscent of bacterial CKs. Sequence analysis of the whole gene indeed revealed substantial identity with known CKs. The question thus arose whether the P. furiosus enzyme was a CPS or a CK.
Several characteristics indicate that the enzyme is a CPS. The most important observation is the stoichiometry of the reaction: two molecules of ATP were found to be consumed per molecule of CP synthesized—a distinctive feature for CPS—whereas CK from S. faecalis, taken as control, consumed only one. In accord with this result the reaction was inhibited by Ap5A but not by Ap3A, which suggests that the enzyme has two ATP-binding sites located at approximately the same distance as in E. coli CPS (22). Furthermore, the reaction was inhibited by α,β-methylene ATP, suggesting that the reaction proceeds via a bicarbonate-dependent ATPase step indicative of bicarbonate phosphorylation as first demonstrated for E. coli CPS (23). Again this is in accord with the stoichiometric analysis which showed that in the absence of ammonium the enzyme consumed ATP at half the rate observed in the presence of this substrate. CK from S. faecalis can also synthesize carboxyphosphate in the absence of ammonium but at a considerably lower rate (3). The data thus indicate that the enzyme we have described is related to CK but functions like a CPS.
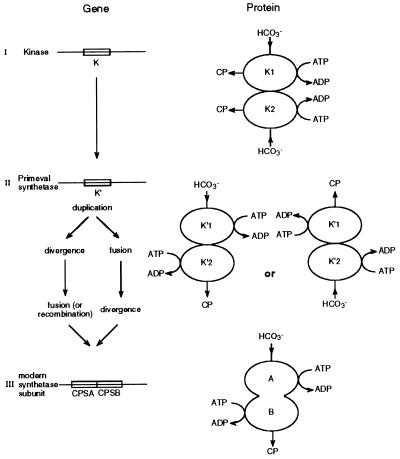
As mentioned in the Introduction, Nyunoya and Lusty (5) and Rubio (13) proposed that the ancestral gene for the synthetase subunits (or domains) of modern CPSs was a CK gene that went through a process of duplication followed by specialization of each copy into one of the two ATP-dependent steps of the reaction catalyzed by CPS. A fusion event would have produced the dual composite structure of modern synthetases, the so-called CPS.A and CPS.B subdomains. Further accretion with a glutaminase would have produced the present glutamine-dependent CPS (14). Our results suggest a somewhat different pathway for the evolution of modern synthetases from the primeval kinase. Indeed, the fact that the P. furiosus enzyme catalyzes the synthesis of CP and is a homodimeric molecule suggests the interesting possibility that either one of its identical subunits can carry out both of the two ATP-dependent reactions: formation of carboxyphosphate and synthesis of CP from carbamate. Once a particular subunit would have synthesized carboxyphosphate, the second one would become committed to the synthesis of CP. This is in striking agreement with the functional model proposed by Guy and Evans (27) on the basis of the observation that homodimers of either the CPS.A or CPS.B subdomains from E. coli and mammalian CPSs, when coupled to the glutaminase subunit, can carry out the complete set of reactions for CP synthesis. Our hypothesis thus assumes that the first step in the evolution of CPS from CK consisted in mutations of a primeval CK gene giving a product similar to the enzyme described here. These mutations would have conferred to the CK polypeptide the dual capacity which is now functionally divided between the CPS.A and CPS.B subdomains of the synthetase subunit. From the thermodynamic point of view such a conversion would have constituted a dramatic improvement because the standard Gibbs free energy changes for CP synthesis catalyzed by CK and CPS are 8.9 kJ/mol and −23.2 kJ/mol, respectively (28). It would not be surprising, therefore, if it had been favored by natural selection. In the scheme presented in Fig. 5 it is, therefore, a primeval synthetase gene and not a primeval CK gene that would have undergone duplication followed by specialization. When considering this evolutionary scheme it is important to note the following points: (i) CK is already capable of carboxyphosphate formation, though at a low rate (3); (ii) from the recently determined three-dimensional structure of E. coli CPS (29), the relative disposition of the two halves of the large subunit suggests a homodimeric origin in keeping with earlier biochemical observations (12, 13); and (iii) as our hypothesis would predict, CK itself, as reported here, is inhibited by Ap5A.
Figure 5.
Scheme illustrating the hypothesis presented in this paper for the evolution of the modern CPS synthetase subunit from a dimeric CK (K). The nitrogen donor (NH3) is not represented nor the glutaminase subunit which, in a later step, is assumed to have combined with the synthetase subunit, conferring upon the enzyme the ability to use glutamine as nitrogen donor. During the transition between steps II and III, fusion (or recombination) joining the primeval synthetase gene (K′) may have preceded divergent evolution of the two copies of the primeval synthetase gene (K′) toward subdomains CPS.A and CPS.B.
Fusion between the primeval synthetase duplicates could have either preceded or followed specialization toward subdomains CPS.A and CPS.B. Furthermore, it is possible that some existing microorganisms still harbor separate genes homologous to the two subdomains. M. jannaschii appears to be a case in point because it harbors two genes reported as carB in its genome (20). After close inspection of the cognate sequences we found that each of these genes, about half the length of E. coli carB, appears homologous to one of the subdomains of the bacterial synthetase, the strongest similarities being observed with Bacillus caldolyticus arginine- and pyrimidine-specific CPSs. The properties of the enzyme have not yet been studied, however. It may well be that it was the possibility of developing the allosteric mechanisms known to control CP synthesis in modern CPSs that provided a selective advantage for the advent of a fused protein. Indeed, the analysis of E. coli synthetase shows that the two subdomains respectively responsible for the synthesis of carboxyphosphate and CP are linked to a C-terminal region in which the main function is the regulation of the enzyme by various effectors (12, 13); furthermore, biochemical studies already suggested that the two halves of the synthetase formed a pseudohomodimer bringing the N-terminal moiety close to the regulatory domain (12, 13). This structural pattern suggests that allosteric regulation is a feature that was added to a pre-existing homo- or already pseudo-homodimer formed by the duplication of the primeval synthetase gene postulated in our hypothesis. The challenge is now to determine what kind of modifications may have converted a dimeric CK into a primeval synthetase and if it will be possible to reproduce these events in the laboratory. Comparisons between crystal structures of Pyrococcus CPS and CPS.A or CPS.B dimers would be of great interest in this respect.
Our original report of an ammonia-dependent CPS in P. furiosus (15) was corroborated by the description of a similar enzyme in Pyrococcus abyssi (30). The enzymatic data are convergent as far as the stoichiometry of the reaction and the effects of ATP analogs are concerned, except that the inhibition by AP5A was shown here not to be specific for CPS but to affect CK as well. However, the native enzyme of P. abyssi was reported to be a monomer of Mr 50,700. This result is intriguing because the stoichiometry of the reaction would imply that the single subunit already possesses two ATP-binding sites; it is in contradiction with the evolutionary schemes discussed above. We feel, however, that the question of the quaternary structure of P. abyssi CPS has to be reconsidered. Indeed, the reported Mr was obtained by size-exclusion chromatography on a Sepharose 6B column close to the limit of applicability of this technique. Moreover, in our hands the elution profiles of P. furiosus and P. abyssi CPS from a Superose P12 column were indistinguishable, suggesting that the two enzymes have the same Mr and that P. abyssi CPS also is a dimer (unpublished results from this laboratory). Cloning and sequence analysis of the P. abyssi gene will indicate if the two enzymes are homologous.
Considering the effects exerted by various ligands on different CPSs, the largest differences observed between the two enzymes concern the effect of temperature: P. abyssi CPS appears quite sensitive to a variety of nucleotides at 37°C, whereas P. furiosus CPS exhibits a slight sensitivity toward a few nucleotides (AMP, CTP, ITP) at low and high temperatures. The greater sensitivity of P. abyssi CPS at 37°C was ascribed to the negative influence of temperature on ionic interactions (30). The physiological meaning of those results is not clear because both P. furiosus and P. abyssi fail to grow at temperatures below 65°C. At any rate, from the genetic information encoded by cpkA we would not expect the cognate enzyme to be regulated like E. coli CPS because the relevant allosteric determinants are absent. As suggested above, the advent of CPS allostery may have required the formation of a duplicate genetic structure.
P. furiosus CPS is strikingly thermostable; as much as 50% activity being retained after 1-h exposure of purified enzyme at 100°C or 3 h at 95°C. This stability is concordant with that of P. furiosus OTC (15). In the absence of a crystal structure, comments on the molecular basis for thermostability must remain limited. The absence of cysteine residues with respect to known CKs (Fig. 4) is however noteworthy.
We propose to use the symbol cpkA to designate the gene for the CK-like CPS of P. furiosus and for possible homologues, such as in P. abyssi. The symbols car, cpa, and cpu have already been attributed to different types of CPSs genes (31, 32) and arcC refers to the CK gene in P. aeruginosa (7). Considering that “enzymes are classified and named according to the reaction they catalyze and taking into account only the observed chemical change produced by the complete enzyme” (Enzyme Nomenclature, 1984, Academic Press, New York, p. 4), we propose to refer to Pyrococcus CPS as a carbamoyl phosphate synthetase (ammonia) (EC 6.3.4.16).
Isotopic competition experiments suggest that P. furiosus OTC and CPS form a complex that channels the thermolabile CP in vivo (15). The three-dimensional structure of P. furiosus OTC was determined recently (V. Villeret, B. Clantin, C. Tricot, C.L., M.R., V. Stalon, N.G. & J. Van Beeumen, unpublished data). The work reported in this paper constitutes another step toward the characterization of this complex. Both genes could be efficiently and faithfully expressed in Saccharomyces cerevisae (ref. 33; and unpublished experiments concerning CPS). This property offers the prospect of analyzing the specificity of interaction between the two proteins by the yeast two-hybrid system (34).
Acknowledgments
We thank D. Gigot for performing chromatographic experiments, S. Delannay for supplying us with purified E. coli CPS, M. Demarez for the production of large amounts of P. furiosus cells, and P. Falmagne and R. Wattiez at the University of Mons-Hainaut for N-terminal amino acid sequences determination. This work was supported by grants from the Belgian Fund for Joint Basic Research, by the European Community Programme Biotechnology, and by the Vlaamse Actieprogramma Biotechnologie.
ABBREVIATIONS
- CPS
carbamoyl phosphate synthetase
- CK
carbamate kinase
- CP
carbamoyl phosphate
- OTC
ornithine transcarbamylase
- Ap3A
P1.P3 di-(adenosine-5′)-triphosphate
- Ap5A
P1.P5 di-(adenosine-5′)-pentaphosphate
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. Y09829).
References
- 1.Meister A. Adv Enzymol. 1989;55:315–374. doi: 10.1002/9780470123089.ch7. [DOI] [PubMed] [Google Scholar]
- 2.Campbell J W, Anderson P M. In: Biochemistry and Molecular Biology of Fishes. Hochachka P W, Mommsen T P, editors. Amsterdam: Elsevier; 1991. pp. 43–76. [Google Scholar]
- 3.Jones M E. Annu Rev Biochem. 1965;34:381–417. doi: 10.1146/annurev.bi.34.070165.002121. [DOI] [PubMed] [Google Scholar]
- 4.Anderson P M, Meister A. Biochemistry. 1966;5:3157–3163. doi: 10.1021/bi00874a012. [DOI] [PubMed] [Google Scholar]
- 5.Nyunoya H, Lusty C J. Proc Natl Acad Sci USA. 1983;80:4629–4633. doi: 10.1073/pnas.80.15.4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marina A, Bravo J, Fita I, Rubio V. J Mol Biol. 1994;235:1345–1347. doi: 10.1006/jmbi.1994.1088. [DOI] [PubMed] [Google Scholar]
- 7.Baur H, Luethi E, Stalon V, Mercenier A, Haas D. Eur J Biochem. 1989;179:53–60. doi: 10.1111/j.1432-1033.1989.tb14520.x. [DOI] [PubMed] [Google Scholar]
- 8.Boettcher B R, Meister A. J Biol Chem. 1980;255:7129–7133. [PubMed] [Google Scholar]
- 9.Post L E, Post D J, Raushel F M. J Biol Chem. 1990;265:7742–7747. [PubMed] [Google Scholar]
- 10.Alonso E, Cervera J, Garcia-Espana A, Bendala E, Rubio V. J Biol Chem. 1992;267:4524–4532. [PubMed] [Google Scholar]
- 11.Guillou F, Liao M, Garcia-Espana A, Lusty C J. Biochemistry. 1992;31:1656–1664. doi: 10.1021/bi00121a012. [DOI] [PubMed] [Google Scholar]
- 12.Rubio V, Cervera J, Lusty C J, Bnedala E, Britton H G. Biochemistry. 1991;30:1068–1075. doi: 10.1021/bi00218a027. [DOI] [PubMed] [Google Scholar]
- 13.Rubio V. Biochem Soc Trans. 1993;21:198–202. doi: 10.1042/bst0210198. [DOI] [PubMed] [Google Scholar]
- 14.Hong J, Wilmar L, Salo C, Lusty J, Anderson P M. J Mol Biol. 1994;243:131–140. doi: 10.1006/jmbi.1994.1638. [DOI] [PubMed] [Google Scholar]
- 15.Legrain C, Demarez M, Glansdorff N, Piérard A. Microbiology. 1995;141:1093–1099. doi: 10.1099/13500872-141-5-1093. [DOI] [PubMed] [Google Scholar]
- 16.Fiala G, Stetter K O. Arch Microbiol. 1986;145:56–61. [Google Scholar]
- 17.Lowry O H, Rosebrough N J, Farr A L, Randall R J. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 18.Miran S G, Chang S H, Raushel F M. Biochemistry. 1991;30:7901–7907. doi: 10.1021/bi00246a005. [DOI] [PubMed] [Google Scholar]
- 19.Eeles R A, Stamps A C. In: Polymerase Chain Reaction (PCR). The Technique and Its Application. Kerkaporta C, editor. Austin, TX: Landes; 1993. pp. 65–68. [Google Scholar]
- 20.Bult C J, White O, Olsen G J, Zhou L X, Fleischmonn R D, et al. Science. 1996;273:1017–1140. [Google Scholar]
- 21.Anderson P M, Meister A. Biochemistry. 1965;4:2803–2809. doi: 10.1021/bi00888a034. [DOI] [PubMed] [Google Scholar]
- 22.Powers S G, Griffith O W, Meister A. J Biol Chem. 1977;252:3558–3560. [PubMed] [Google Scholar]
- 23.Powers S G, Meister A. J Biol Chem. 1978;253:1258–1265. [PubMed] [Google Scholar]
- 24.Thomm M. FEMS Microbiol Rev. 1996;18:159–171. doi: 10.1111/j.1574-6976.1996.tb00234.x. [DOI] [PubMed] [Google Scholar]
- 25.Hain J, Reiter W-D, Hüdepohl U, Zillig W. Nucleic Acids Res. 1992;20:5423–5428. doi: 10.1093/nar/20.20.5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reiter W-D, Palm P, Zillig W. Nucleic Acids Res. 1988;16:2445–2459. doi: 10.1093/nar/16.6.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guy H I, Evans D R. J Biol Chem. 1996;271:13762–13769. doi: 10.1074/jbc.271.23.13762. [DOI] [PubMed] [Google Scholar]
- 28.Rubio R. Biochem Soc Trans. 1993;21:198–202. doi: 10.1042/bst0210198. [DOI] [PubMed] [Google Scholar]
- 29.Thoden J B, Holden H M, Wesenberg G, Raushel F M, Raymant I. Biochemistry. 1997;36:6305–6316. doi: 10.1021/bi970503q. [DOI] [PubMed] [Google Scholar]
- 30.Purcarea C, Simon V, Prieur D, Hervé G. Eur J Biochem. 1996;236:189–199. doi: 10.1111/j.1432-1033.1996.00189.x. [DOI] [PubMed] [Google Scholar]
- 31.Cunin R, Glansdorff N, Piérard A, Stalon V. Microbiol Rev. 1986;50:314–352. doi: 10.1128/mr.50.3.314-352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis R H. Microbiol Rev. 1986;50:280–313. doi: 10.1128/mr.50.3.280-313.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roovers M, Hethke C, Legrain C, Thomm M, Glansdorff N. Eur J Biochem. 1997;247:1038–1045. doi: 10.1111/j.1432-1033.1997.01038.x. [DOI] [PubMed] [Google Scholar]
- 34.Fields S, Song O. Nature (London) 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]