Abstract
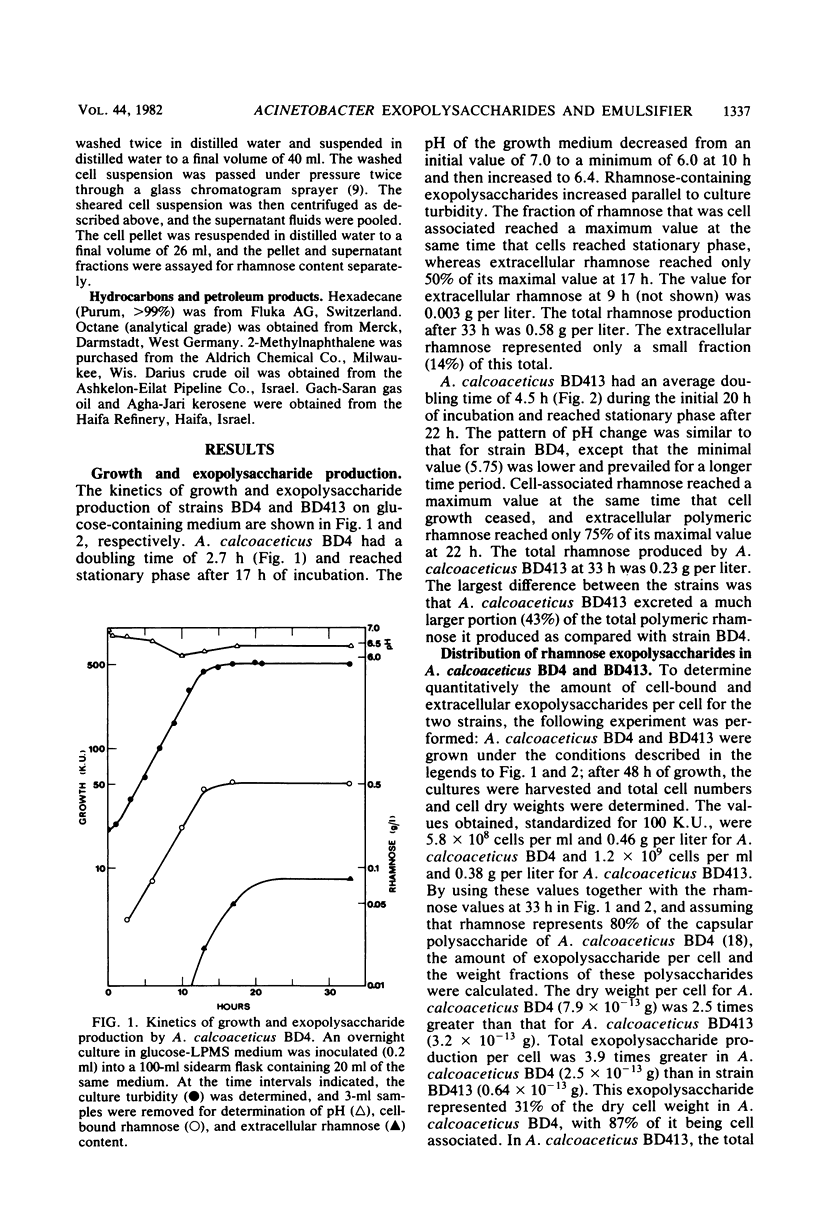
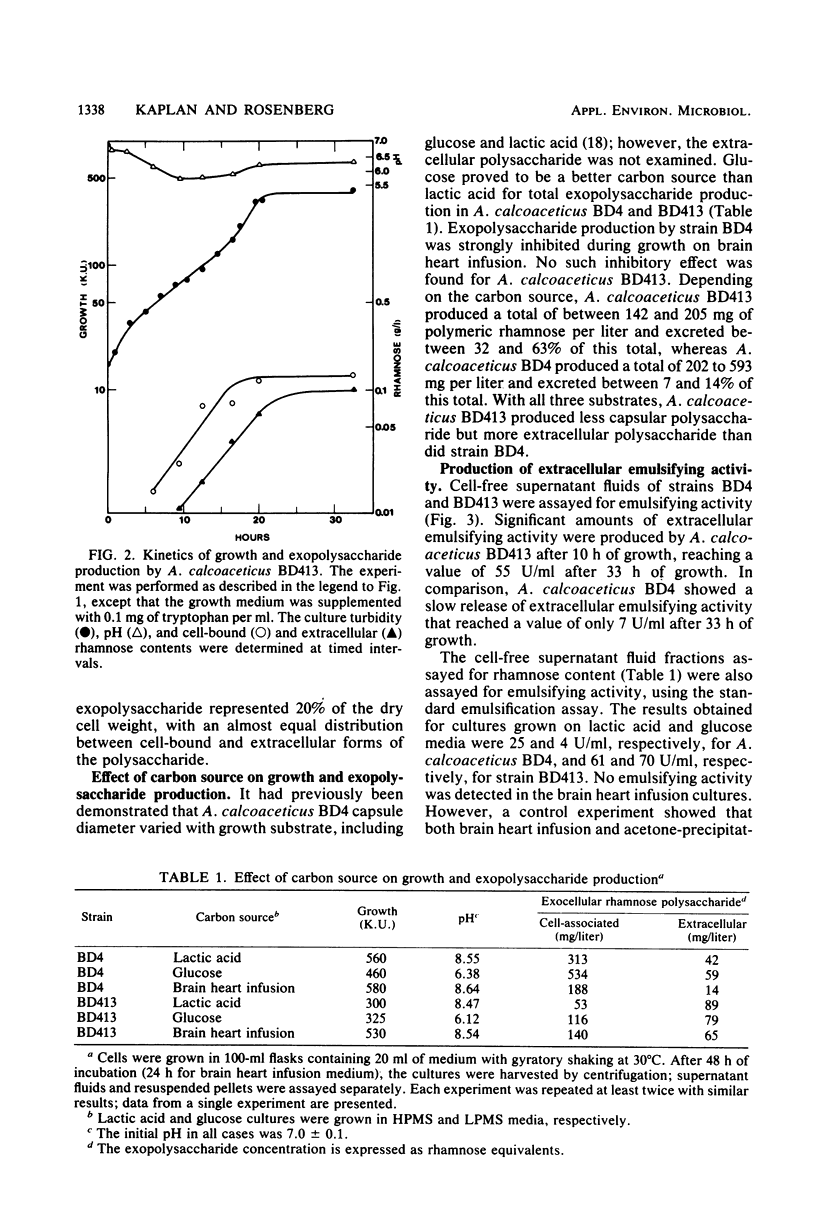
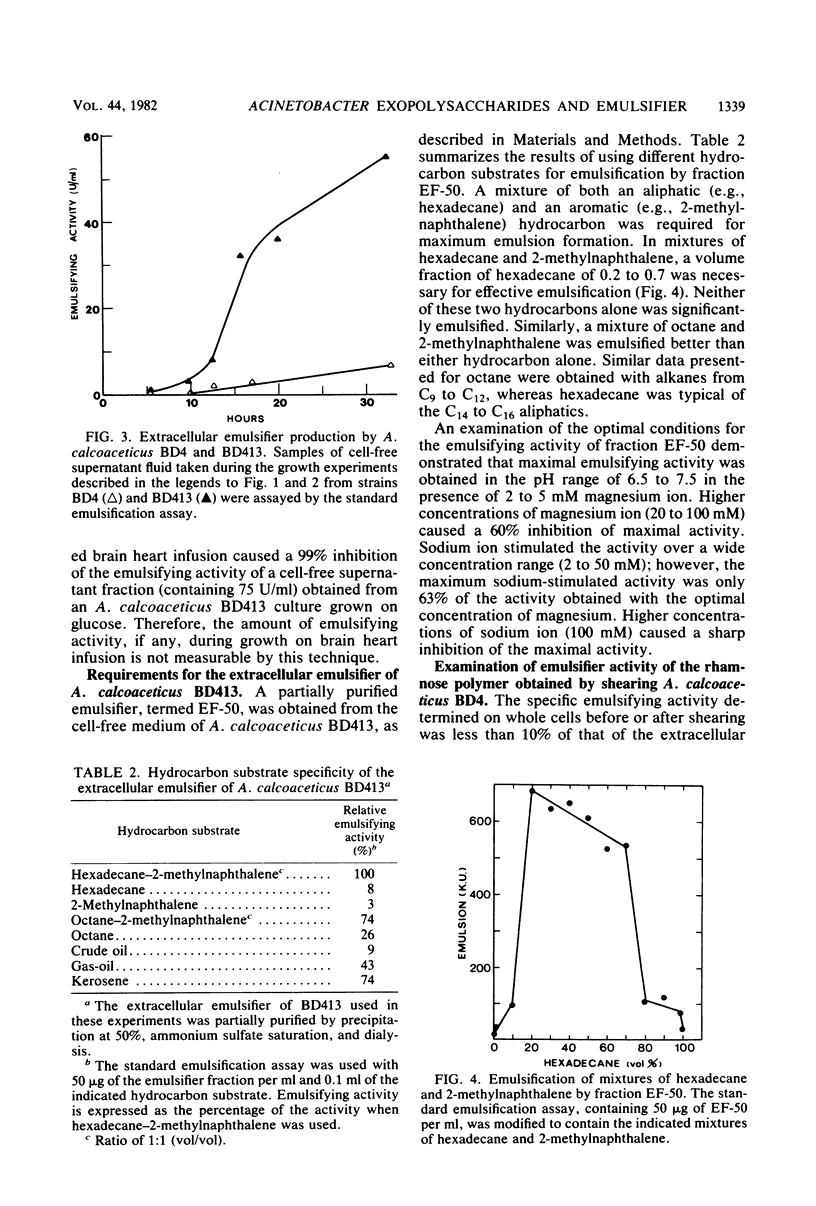
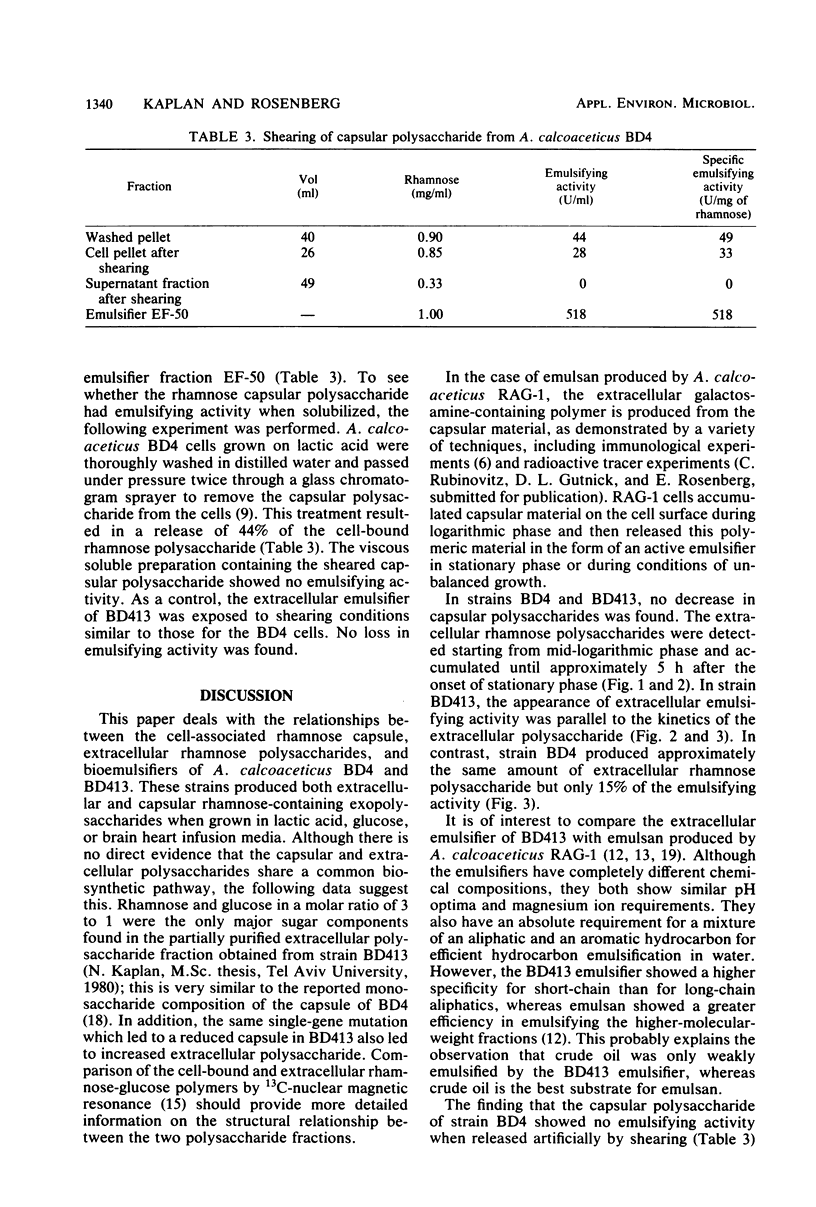
The heavily encapsulated Acinetobacter calcoaceticus BD4 and the “miniencapsulated” single-step mutant A. calcoaceticus BD413 produced extracellular polysaccharides in addition to the capsular material. The molar ratio of rhamnose to glucose (3:1) in the extracellular BD413 polysaccharide fraction was similar to the composition of the capsular material. In both strains, the increase in capsular polysaccharide was parallel to cell growth and remained constant in stationary phase. The extracellular polysaccharides were detected starting from mid-logarithmic phase and continued to accumulate in the growth medium for 5 to 8 h after the onset of stationary phase. Strain BD413 produced one-fourth the total rhamnose exopolysaccharide per cell that strain BD4 did. Depending on the growth medium, 32 to 63% of the rhamnose polysaccharide produced by strain BD413 was extracellular, whereas in strain BD4 only 7 to 14% was extracellular. In all cases, strain BD413 produced more extracellular rhamnose polysaccharide than strain BD4 did. In glucose medium, strain BD413 also produced approximately 10 times more extracellular emulsifying activity than strain BD4 did. The isolated capsular polysaccharide obtained after shearing of BD4 cells showed no emulsifying activity. Thus, strain BD413 either produces a modified extracellular polysaccharide or excretes an additional substance(s) that is responsible for the emulsifying activity. Emulsions induced by the ammonium sulfate-precipitated BD413 extracellular emulsifier require the presence of magnesium ion and a mixture of an aliphatic and an aromatic hydrocarbon.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer M. E., Thurow H. Polysaccharide capsule of Escherichia coli: microscope study of its size, structure, and sites of synthesis. J Bacteriol. 1977 May;130(2):911–936. doi: 10.1128/jb.130.2.911-936.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky I., Gutnick D. L., Rosenberg E. Emulsifier of Arthrobacter RAG-1: determination of emulsifier-bound fatty acids. FEBS Lett. 1979 May 1;101(1):175–178. doi: 10.1016/0014-5793(79)81320-4. [DOI] [PubMed] [Google Scholar]
- Brooks K., Sodeman T. Clinical studies on a transformation test for identification of Acinetobacter (Mima and Herellea). Appl Microbiol. 1974 Jun;27(6):1023–1026. doi: 10.1128/am.27.6.1023-1026.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUDMAN W. F., WILKINSON J. F. The composition of the extracellular polysaccharides of Aerobacter-Klebsiella strains. Biochem J. 1956 Feb;62(2):289–295. doi: 10.1042/bj0620289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungerer D., Jann K., Jann B., Orskov F., Orskov I. Immunochemistry of K antigens of Escherichia coli. 4. The K antigen of E. coli O 9:K30:H12. Eur J Biochem. 1967 Jul;2(1):115–126. doi: 10.1111/j.1432-1033.1967.tb00115.x. [DOI] [PubMed] [Google Scholar]
- JUNI E., HEYM G. A. PATHWAYS FOR BIOSYNTHESIS OF A BACTERIAL CAPSULAR POLYSACCHARIDE. IV. CAPSULE RESYNTHESIS BY DECAPSULATED RESTING-CELL SUSPENSIONS. J Bacteriol. 1964 Feb;87:461–467. doi: 10.1002/path.1700870234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juni E. Interspecies transformation of Acinetobacter: genetic evidence for a ubiquitous genus. J Bacteriol. 1972 Nov;112(2):917–931. doi: 10.1128/jb.112.2.917-931.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juni E., Janik A. Transformation of Acinetobacter calco-aceticus (Bacterium anitratum). J Bacteriol. 1969 Apr;98(1):281–288. doi: 10.1128/jb.98.1.281-288.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisfeld A., Rosenberg E., Gutnick D. Microbial degradation of crude oil: factors affecting the dispersion in sea water by mixed and pure cultures. Appl Microbiol. 1972 Sep;24(3):363–368. doi: 10.1128/am.24.3.363-368.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg E., Perry A., Gibson D. T., Gutnick D. L. Emulsifier of Arthrobacter RAG-1: specificity of hydrocarbon substrate. Appl Environ Microbiol. 1979 Mar;37(3):409–413. doi: 10.1128/aem.37.3.409-413.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg E., Zuckerberg A., Rubinovitz C., Gutnick D. L. Emulsifier of Arthrobacter RAG-1: isolation and emulsifying properties. Appl Environ Microbiol. 1979 Mar;37(3):402–408. doi: 10.1128/aem.37.3.402-408.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawula R. V., Crawford I. P. Mapping of the tryptophan genes of Acinetobacter calcoaceticus by transformation. J Bacteriol. 1972 Nov;112(2):797–805. doi: 10.1128/jb.112.2.797-805.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR W. H., JUNI E. Pathways for biosynthesis of a bacterial capsular polysaccharide. I. Characterization of the organism and polysaccharide. J Bacteriol. 1961 May;81:688–693. doi: 10.1128/jb.81.5.688-693.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerberg A., Diver A., Peeri Z., Gutnick D. L., Rosenberg E. Emulsifier of Arthrobacter RAG-1: chemical and physical properties. Appl Environ Microbiol. 1979 Mar;37(3):414–420. doi: 10.1128/aem.37.3.414-420.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


